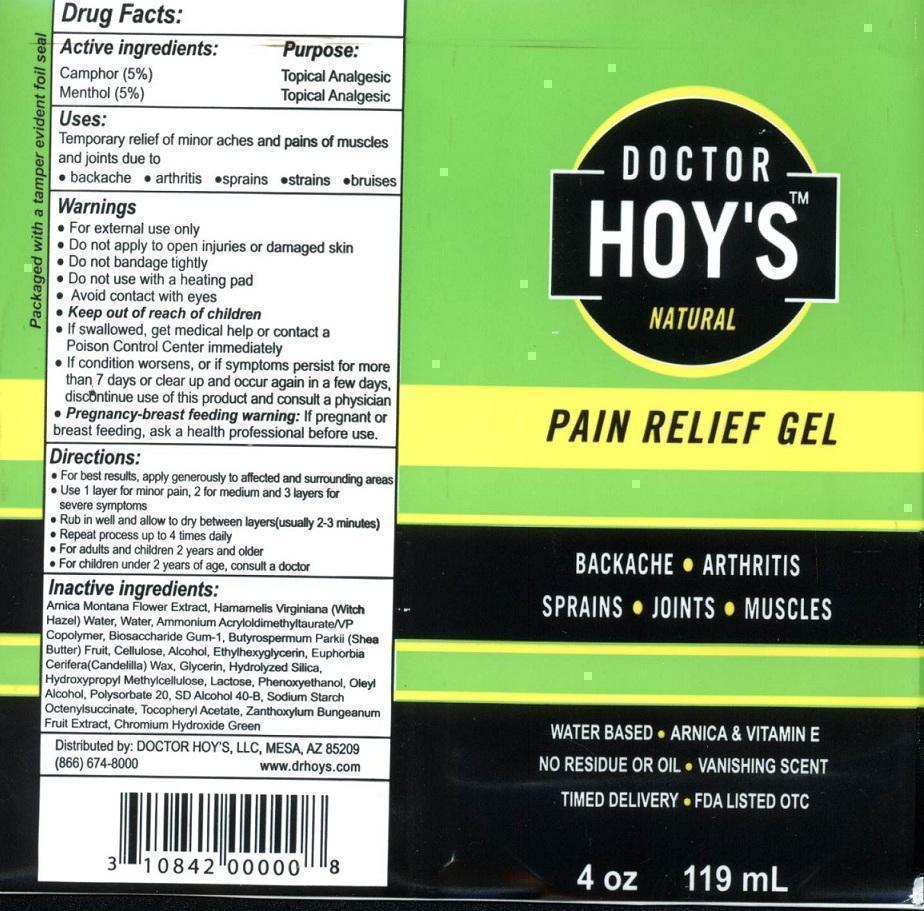
DOCTOR HOYS NATURAL PAIN RELIEF- camphor menthol gel
DOCTOR HOY'S, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Doctor Hoys Pain Relief Gel
USES:
TEMPORARY RELIEF OF MINOR ACHES AND PAINS OF MUSCLES AND JOINTS DUE TO
- BACKACHE
- ARTHRITIS
- SPRAINS
- STRAINS
- BRUISES
WARNINGS
- FOR EXTERNAL USE ONLY
- DO NOT APPLY TO OPEN INJURIES OR DAMAGED SKIN
- DO NOT BANDAGE TIGHTLY
- DO NOT USE WITH A HEATING PAD
- AVOID CONTACT WITH EYES
- KEEP OUT OF REACH OF CHILDREN
- IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER IMMEDIATELY
- IF CONDITION WORSENS, OR IF SYMPTOMS PERSIST FOR MORE THAN 7 DAYS, DISCONTINUE USE OF THIS PRODUCT AND CONSULT A PHYSICIAN.
-
PREGNANCY-BREAST FEEDING WARNING: IF PREGNANT OR BREAST FEEDING, AS A HEALTH PROFESSIONAL BEFORE USE.
DIRECTIONS
- FOR BEST RESULTS, APPLY GENEROUSLY TO AFFECTED AND SURROUNDING AREAS
- USE 1 LAYER FOR MINOR PAIN, 2 FOR MEDIUM AND 3 LAYERS FOR SEVERE SYMPTOMS
- RUB IN WELL AND ALLOW TO DRY BETWEEN LAYERS (USUALLY 2-3 MINUTES)
- REPEAT PROCESS UP TO 4 TIMES DAILY
- FOR ADULTS AND CHILDREN 2 YEARS AND OLDER
- FOR CHILDREN UNDER 2 YEARS OF AGE, CONSULT A DOCTOR
INACTIVE INGREDIENTS
ARNICA MONTANA FLOWER EXTRACT, HAMAMELIS VIRGINIANA (WITCH HAZEL) WATER, WATER, AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER, BIOSACCHARIDE GUM-1, BUTYROSPERMUM PARKII (SHEA BUTTER) FRUIT, CELLULOSE, ALCOHOL, ETHYLHEXYLGLYCERIN, EUPHORBIA CERIFERA (CANDELILLA) WAX, GLYCERIN, HYDROLYZED SILICA, HYDROXYPROPYL METHYLCELLULOSE, LACTOSE, PHENOXYETHANOL, OLEYL ALCOHOL, POLYSORBATE 20, SD ALCOHOL 40-B, SODIUM STARCH OCTNYLSUCCINATE, TOCOPHERYL ACETATE, ZANTHOXYLUM BUNGEANUM FRUIT EXTRACT, CHROMIUM HYDROXIDE GREEN
| DOCTOR HOYS NATURAL PAIN RELIEF
camphor menthol gel |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - DOCTOR HOY'S, LLC (791882322) |