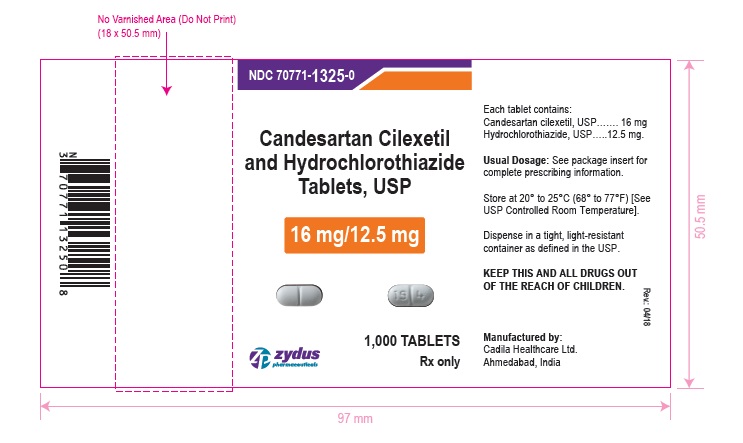
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 70771-1325-0 in bottle of 1000 tablets
Candesartan Cilexetil and Hydrochlorothiazide Tablets USP, 16 mg/12.5 mg
Rx only
1000 tablets
NDC 70771-1326-0 in bottle of 1000 tablets
Candesartan Cilexetil and Hydrochlorothiazide Tablets USP, 32 mg/12.5 mg
Rx only
1000 tablets

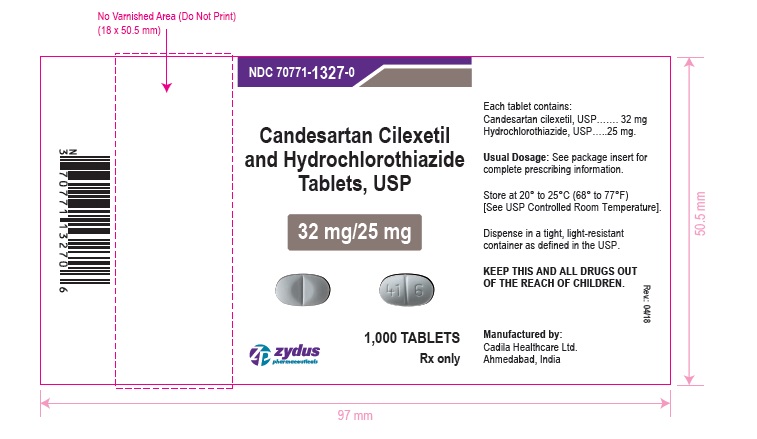
NDC 70771-1327-0 in bottle of 1000 tablets
Candesartan Cilexetil and Hydrochlorothiazide Tablets USP, 32 mg/25 mg
Rx only
1000 tablets