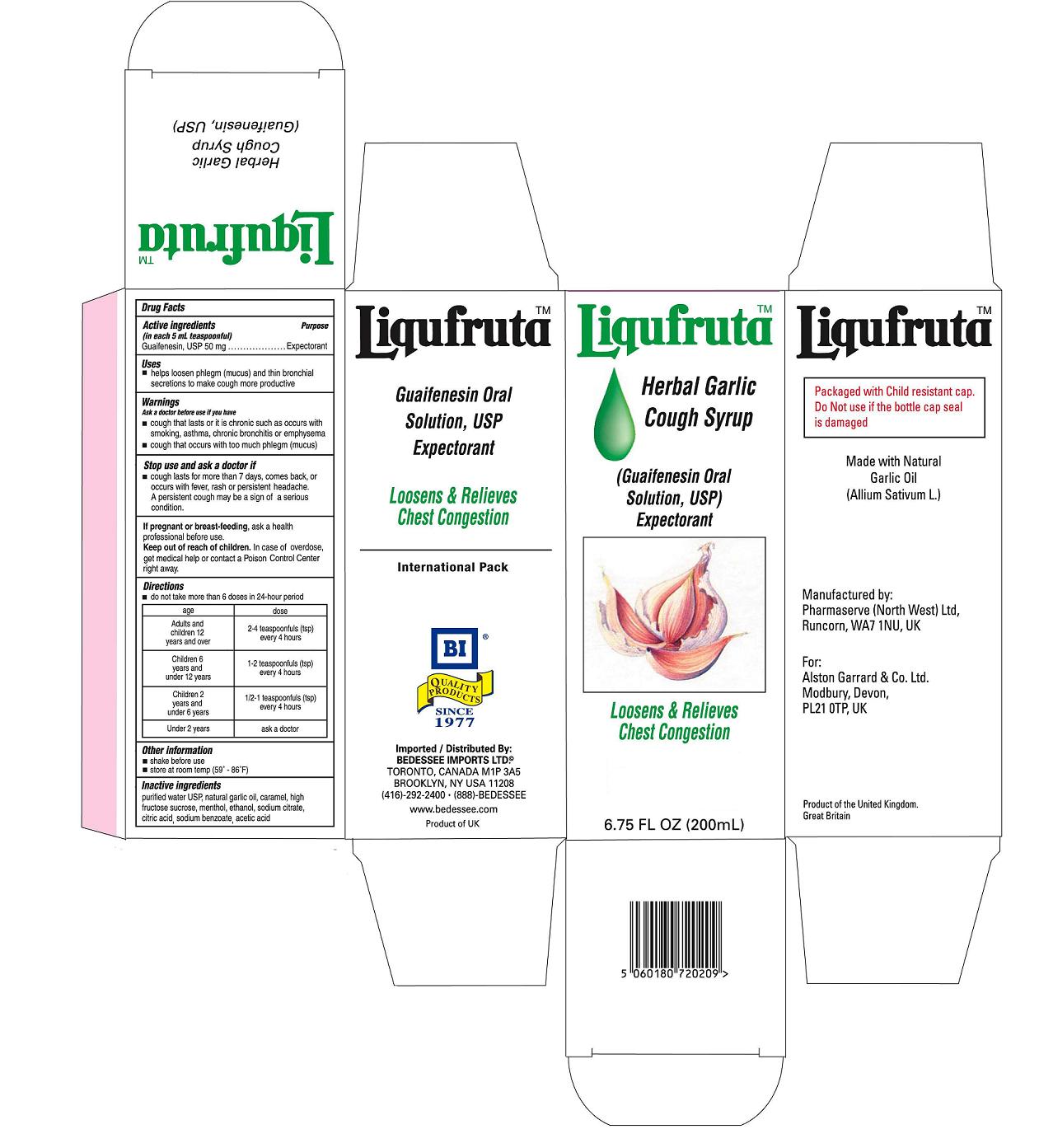
EXPECTORANT -HELPS LOOSEN PHLEGM (MUCUS) AND THIN BRONCHIAL SECRETIONS TO MAKE COUGH MORE PRODUCTIVE
Keep out reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Ask a doctor before use if you have a cough that last or it is chronic such as occurs with smoking, asthma, chronic Bronchitis or emphysema. Stop use and ask doctor if a cough lasts for more than 7 days comes back or occurs when fever, rash or persistent headache. A persistent cough may be a sign of a serious condition.
If pregnant of breast-feeding, ask a health professional before use.
If pregnant of breast-feeding, ask a health professional before use.
do not take more than 6 doses in a 24-hour period
AGE ......................................................DOSE
Adults and Children 12 years and over .......2-4 teaspoonfuls(tsp) every 4 hours
Children 6 years and under 12 years......... 1-2 teaspoonfuls(tsp) every 4 hours
Children 2 years and under 6 years........... 1/2-1 teaspoonfuls(tsp) every 4 hours
Under 2 years ........................................ask a doctor
AGE ......................................................DOSE
Adults and Children 12 years and over .......2-4 teaspoonfuls(tsp) every 4 hours
Children 6 years and under 12 years......... 1-2 teaspoonfuls(tsp) every 4 hours
Children 2 years and under 6 years........... 1/2-1 teaspoonfuls(tsp) every 4 hours
Under 2 years ........................................ask a doctor
Purified water, natural garlic oil,caramel, high fructose sucrose, menthol, ethanol, sodium citrate, citric acid, sodium benzoate, acetic acid