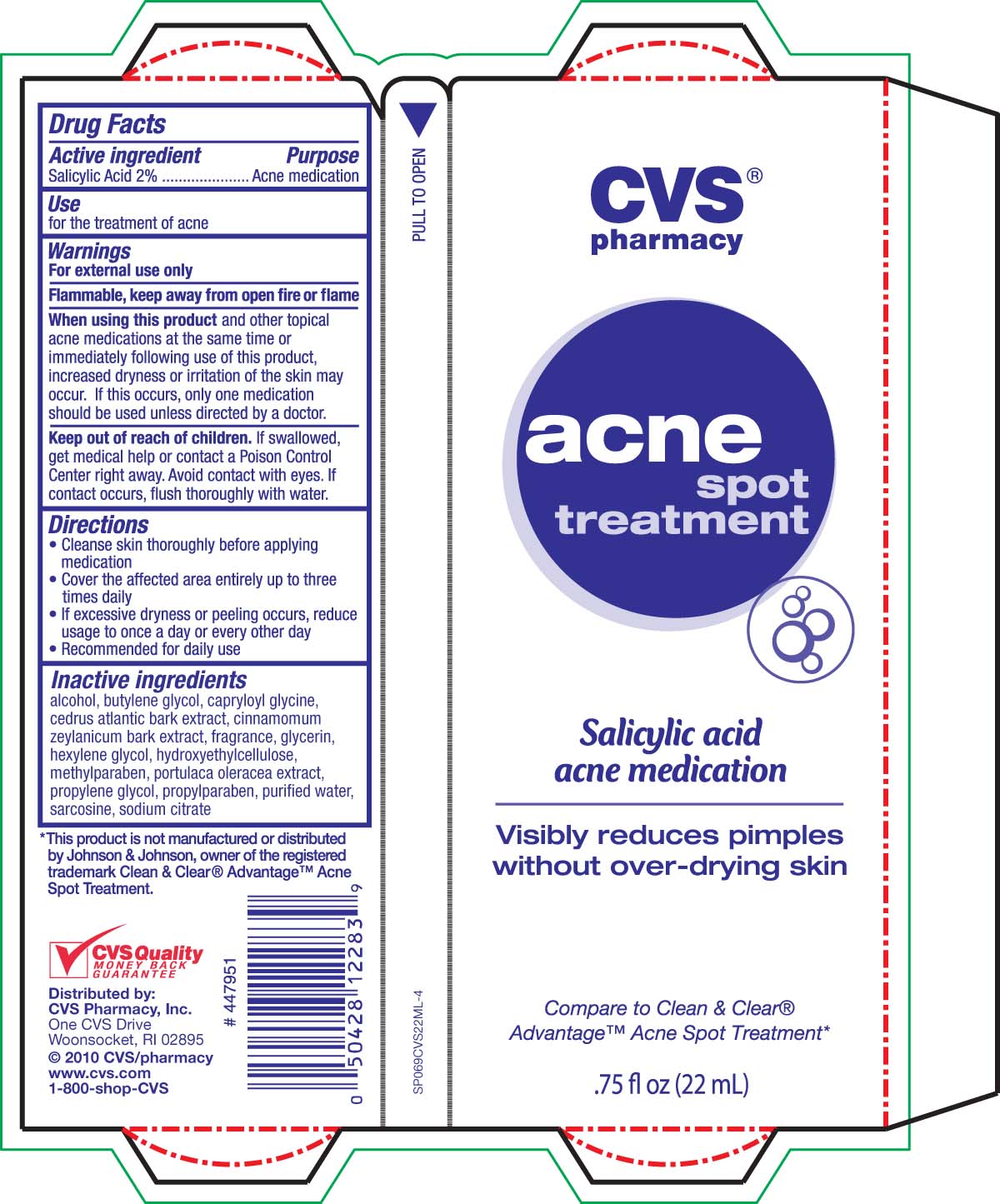
Active ingredient Purpose
Salicylic Acid 2%............................................Acne medication
Salicylic Acid 2%............................................Acne medication
Keep out of reach of children. If swallowed, get medical help or contact a
Poison Control Center right away. Avoid contact with eyes. If contact occurs,
flush thoroughly with water.
Warnings
For external use only
Flammable, keep away from open fire or flame
When using this product and other topical acne medications at the same time
or immediately following use of this product, increased dryness or irritation of the skin
may occur. If this occurs, only one medication should be used unless directed by a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison
Control Center right away. Avoid contact with eyes. If contact occurs, flush thoroughly with water.
For external use only
Flammable, keep away from open fire or flame
When using this product and other topical acne medications at the same time
or immediately following use of this product, increased dryness or irritation of the skin
may occur. If this occurs, only one medication should be used unless directed by a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison
Control Center right away. Avoid contact with eyes. If contact occurs, flush thoroughly with water.
Directions
- Cleanse skin thoroughly before applying medication
- Cover the affected area entirely up to three times daily
- If excessive dryness or peeling occurs, reduce usage to once a day or every other day
- Recommended for daily use
Inactive Ingredients
alcohol, butylene glycol, capryloyl glycine, cedrus atlantic bark extract, cinnamomum
zeylanicum bark extract, fragrance, glycerin, hexylene glycol, hydroxyethylcellulose,
methylparaben, portulica oleracea extract, propylene glycol, propylparaben, purified water,
sarcosine, sodium citrate
alcohol, butylene glycol, capryloyl glycine, cedrus atlantic bark extract, cinnamomum
zeylanicum bark extract, fragrance, glycerin, hexylene glycol, hydroxyethylcellulose,
methylparaben, portulica oleracea extract, propylene glycol, propylparaben, purified water,
sarcosine, sodium citrate