QUENALIN COUGH- diphenhydramine hydrochloride syrup
Qualitest Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
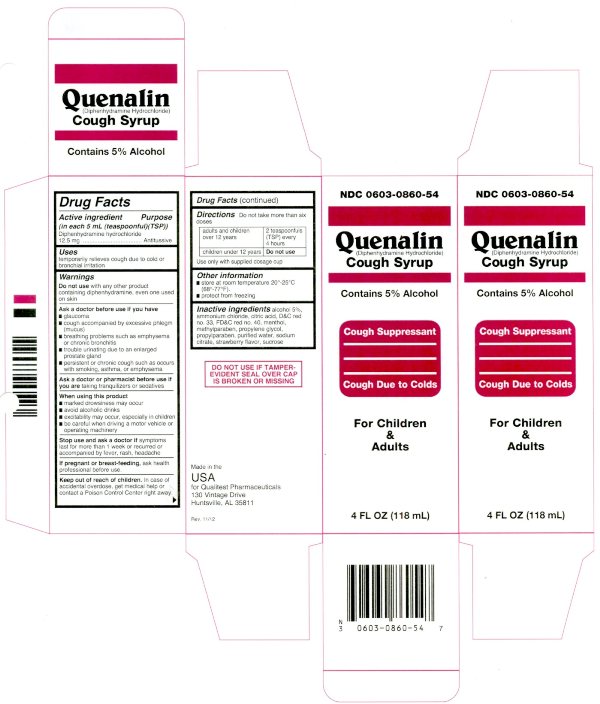
Quenalin Cough Syrup
Warnings
Do not use with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- glaucoma
- cough accompanied by excessive phlegm (mucus)
- breathing problems such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- excitability may occur, especially in children
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
symptoms last for more than 1 week or recurred or accompanied by fever, rash, headache
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Do not take more than six doses
| Adults and children over 12 years | 2 teaspoonfuls(TSP) every 4 hours |
| children under 12 years | DO NOT USE |
Use only with supplied dosage cup
| QUENALIN COUGH
diphenhydramine hydrochloride syrup |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Qualitest Pharmaceuticals (011103059) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Silarx Pharmaceuticals, Inc. | 161630033 | MANUFACTURE(0603-0860) | |
Revised: 11/2016
Document Id: 1985c7d9-b29d-49a3-92f3-653a929fa559
Set id: c8dbe8d6-8816-4aee-a9e1-035a04aa3666
Version: 13
Effective Time: 20161108
Qualitest Pharmaceuticals