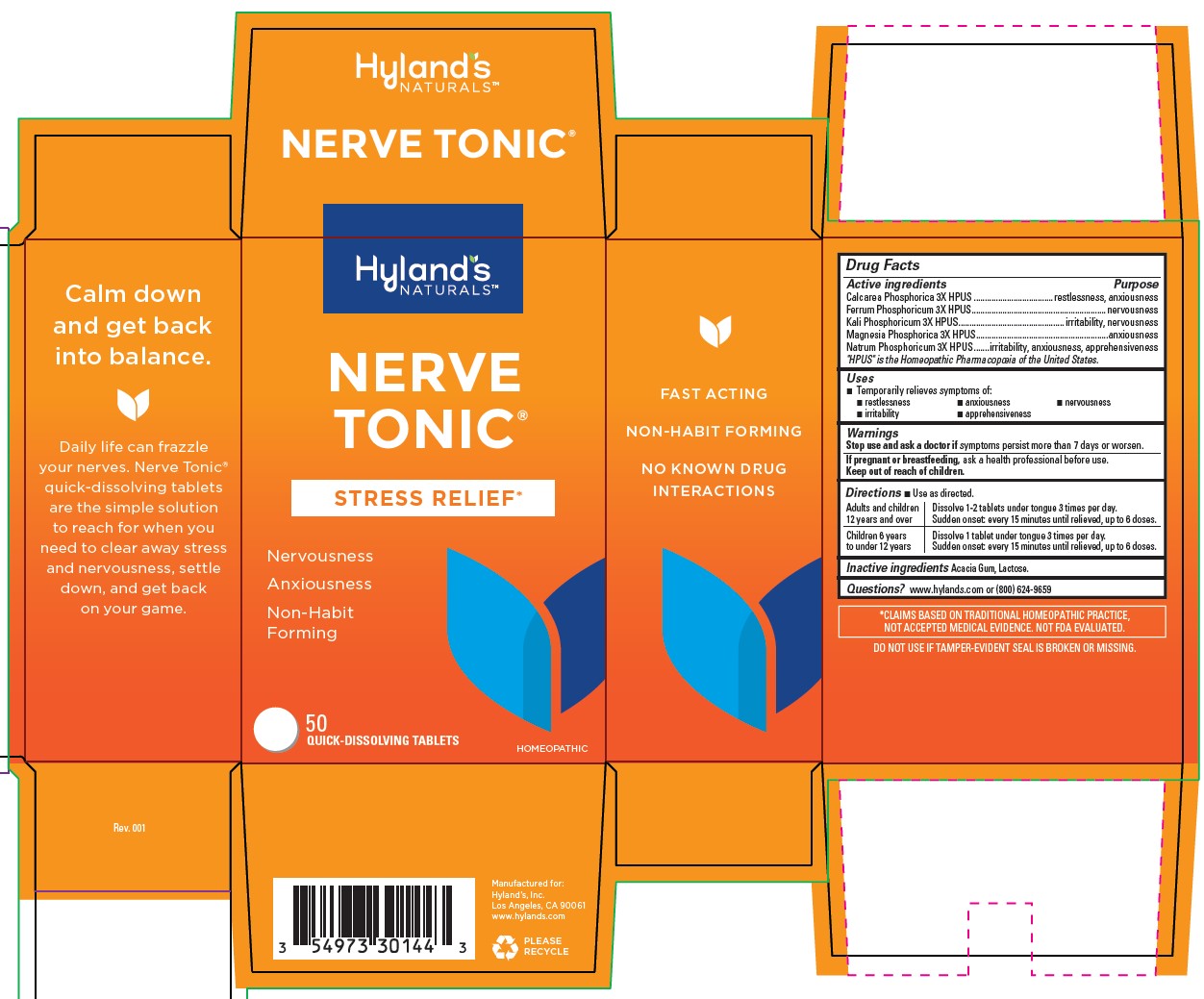
Drug Facts
Active ingredients
|
Active ingredients |
Purpose |
|
Calcarea Phosphorica 3X HPUS |
restlessness, anxiousness |
|
Ferrum Phosphoricum 3X HPUS |
nervousness |
|
Kali Phosphoricum 3X HPUS |
irritability,nervousness |
|
Magnesia Phosphorica 3X HPUS |
anxiousness |
|
Natrum Phosphoricum 3X HPUS |
irritability,apprehensiveness |
“HPUS” indicates that the active ingredients are in the official Homeopathic Pharmacopœia of the United States.
Uses
■ Temporarily relieves the symptoms of: ■ restlessness ■ anxiousness ■ nervousness ■ irritability ■ apprehensiveness
Directions
■ Use as directed.
| Adults and children
12 years and over | Dissolve 1-2 tablets under tongue 3 times per day.
Sudden onset: every 15 minutes until relieved, up to 6 doses. |
| Children 6 years
to under 12 years | Dissolve 1 tablet under tongue 3 times per day.
Sudden onset: every 15 minutes until relieved, up to 6 doses. |