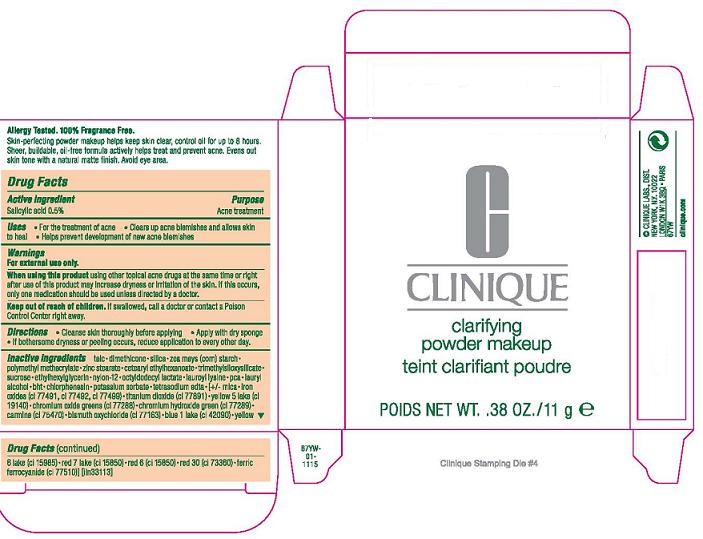
PURPOSE: ACNE TREATMENT
USE: FOR THE TREATMENT OF ACNE
CLEARS UP ACNE BLEMISHES AND ALLOWS SKIN TO HEAL
HELPS PREVENT DEVELOPMENT OF NEW ACNE BLEMISHES
WHEN USING THIS PRODUCT: USING OTHER TOPICAL ACNE DRUGS AT THE SAME TIME OR RIGHT AFTER USE OF THIS PRODUCT MAY INCREASE DRYNESS OR IRRITATION OF THE SKIN. IF THIS OCCURS, ONLY ONE MEDICATION SHOULD BE USED UNLESS DIRECTED BY A DOCTOR.
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS
- CLEANSE SKIN THOROUGHLY BEFORE APPLYING
- COVER THE ENTIRE AFFECTED AREA WITH ONE APPLICATION
- GRADUALLY INCREASE TO 2 OR 3 TIMES DAILY IF NEEDED OR AS DIRECTED BY A DOCTOR
- IF BOTHERSOME DRYNESS OR PEELING OCCURS, REDUCE APPLICATION TO EVERY OTHER DAY.
INACTIVE INGREDIENTS:
talc [] dimethicone [] silica [] zea mays (corn) starch [] polymethyl methacrylate [] zinc stearate [] cetearyl ethylhexanoate [] trimethylsiloxysilicate [] sucrose [] ethylhexylglycerin [] nylon-12 [] octyldodecyl lactate [] lauroyl lysine [] pca [] lauryl alcohol [] bht [] chlorphenesin [] potassium sorbate [] tetrasodium edta [] [+/- mica [] iron oxides (ci 77491, ci 77492, ci 77499) [] titanium dioxide (ci 77891) [] yellow 5 lake (ci 19140) [] chromium oxide greens (ci 77288) [] chromium hydroxide green (ci 77289) [] carmine (ci 75470) [] bismuth oxychloride (ci 77163) [] blue 1 lake (ci 42090) [] yellow 6 lake (ci 15985) [] red 7 lake (ci 15850) [] red 6 (ci 15850) [] red 30 (ci 73360) [] ferric ferrocyanide (ci 77510