Warnings
- For external use only
- FLAMMABLE. Keep away from fire or flame, heat, sparks and sources of static discharge.
Other information
- for additional information, see Safety Data Sheet (SDS)
- for emergency medical information in USA, call 1.800.328.0026
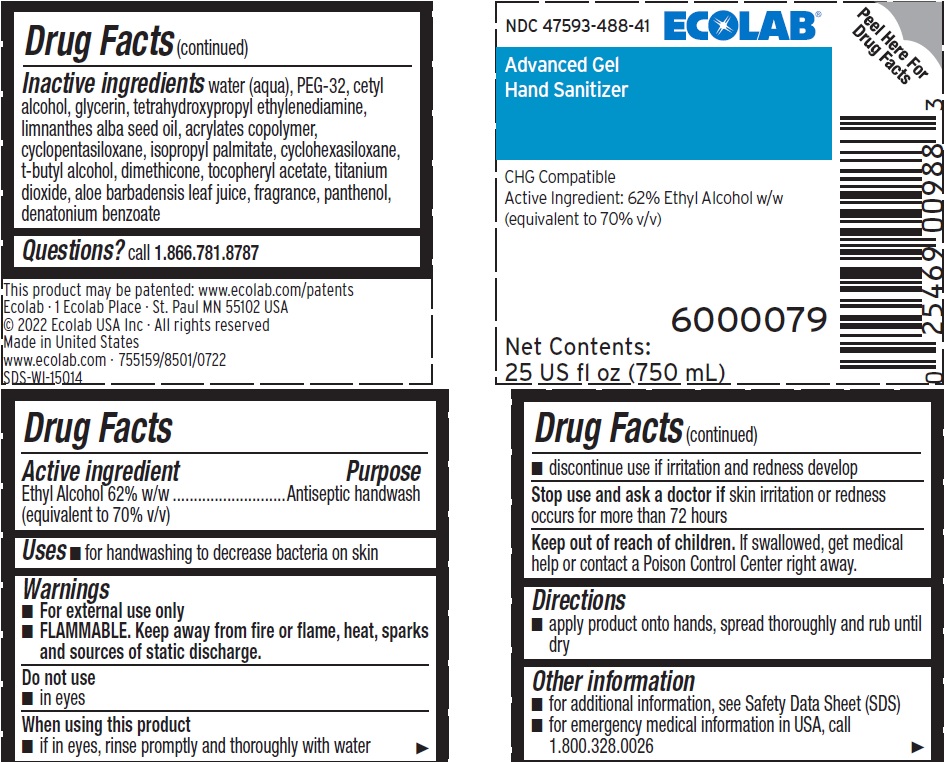
Inactive ingredients Water (Aqua), PEG-32, Cetyl Alcohol, Glycerin, Tetrahydroxypropyl Ethylenediamine, Limnanthes Alba Seed Oil , Acrylates Copolymer, Cyclopentasiloxane, Isopropyl Palmitate, Cyclohexasiloxane, t-Butyl Alcohol, Dimethicone, Tocopheryl Acetate, Titanium Dioxide, Aloe Barbadensis Leaf Juice, fragrance, Panthenol, Denatonium benzoate
Representative label and principal display panel
NDC 47593-488-41 ECOLAB
Advanced Gel
Hand Sanitizer
CHG Compatible
Active Ingredient: 62% Ethyl Alcohol w/w
(equivalent to 70% v/v)
6000079
Net Contents:
25 US fl oz (750 mL)
This product may be patented: www.ecolab.com/patents
Ecolab · 1 Ecolab Place · St. Paul MN 55102 USA
© 2022 Ecolab USA Inc · All rights reserved
Made in United States
www.ecolab.com · 755159/8501/0722
SDS-WI-15014