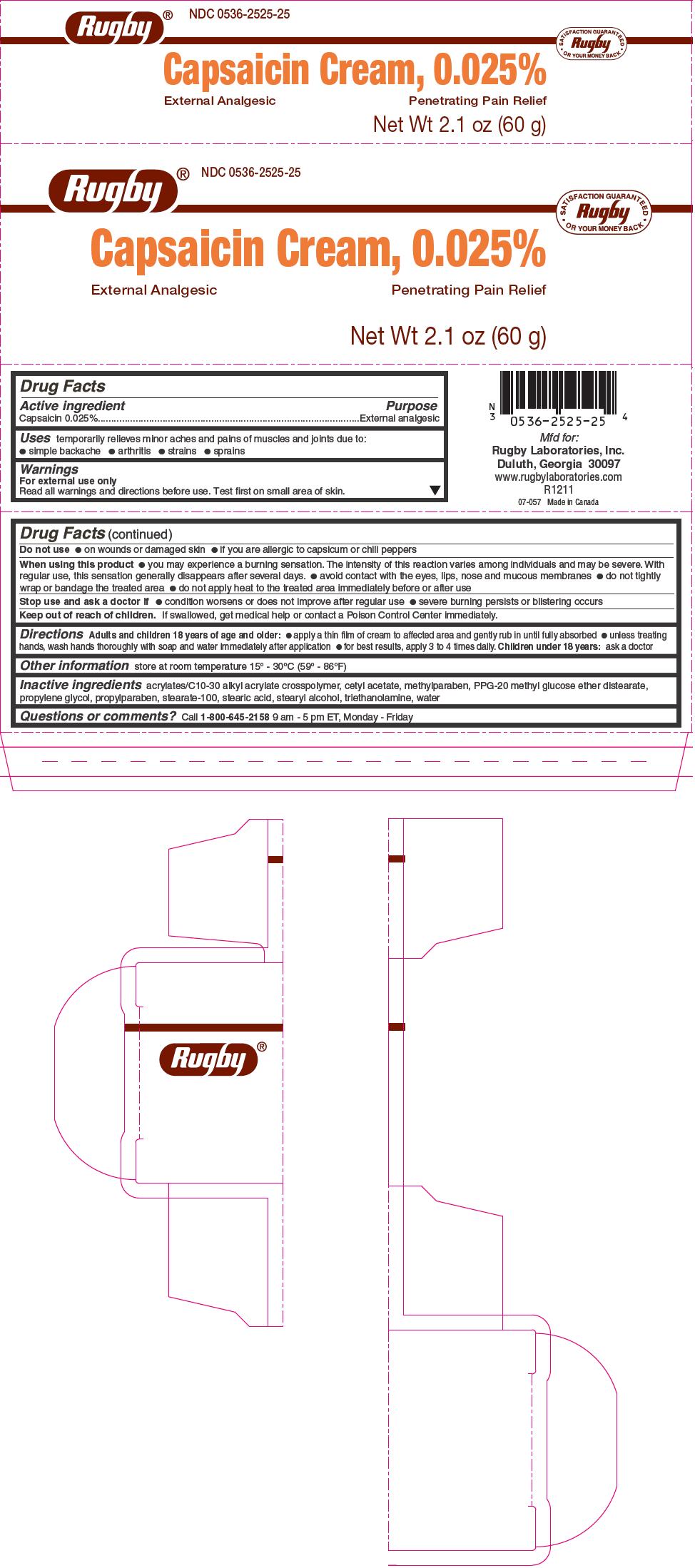
Uses
temporarily relieves minor aches and pains of muscles and joints due to:
- simple backache
- arthritis
- strains
- sprains
Warnings
For external use only
Read all warnings and directions before use. Test first on small area of skin.
Do not use
- on wounds or damaged skin
- if you are allergic to capsicum or chili peppers
When using this product
- you may experience a burning sensation. The intensity of this reaction varies among individuals and may be severe. With regular use, this sensation generally disappears after several days.
- avoid contact with the eyes, lips, nose and mucous membranes
- do not tightly wrap or bandage the treated area
- do not apply heat to the treated area immediately before or after use
Directions
Adults and children 18 years of age and older:
- apply a thin film of cream to affected area and gently rub in until fully absorbed
- unless treating hands, wash hands thoroughly with soap and water immediately after application
- for best results, apply 3 to 4 times daily.
Children under 18 years: ask a doctor