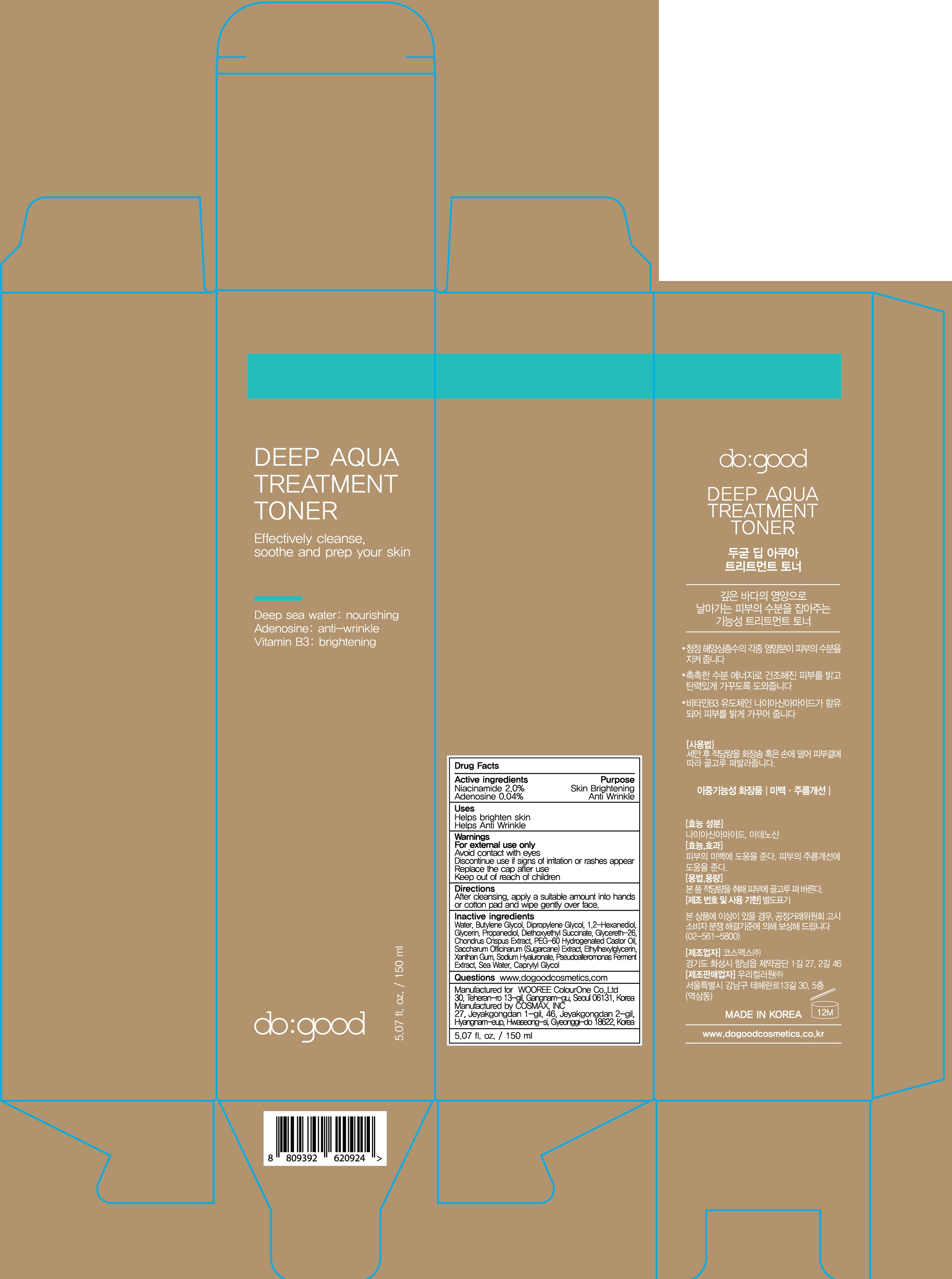
INACTIVE INGREDIENT
Inactive ingredients:
Water, Butylene Glycol, Dipropylene Glycol, 1,2-Hexanediol, Glycerin, Propanediol, Diethoxyethyl Succinate, Glycereth-26, Chondrus Crispus Extract, PEG-60 Hydrogenated Castor Oil, Saccharum Officinarum (Sugarcane) Extract, Ethylhexylglycerin, Xanthan Gum, Sodium Hyaluronate, Pseudoalteromonas Ferment Extract, Sea Water, Caprylyl Glycol
WARNINGS
Warnings:
For external use only.
Avoid contact with eyes.
Discontinue use if signs of irritation or rashes appear.
Replace the cap after use.
Keep out of reach of children