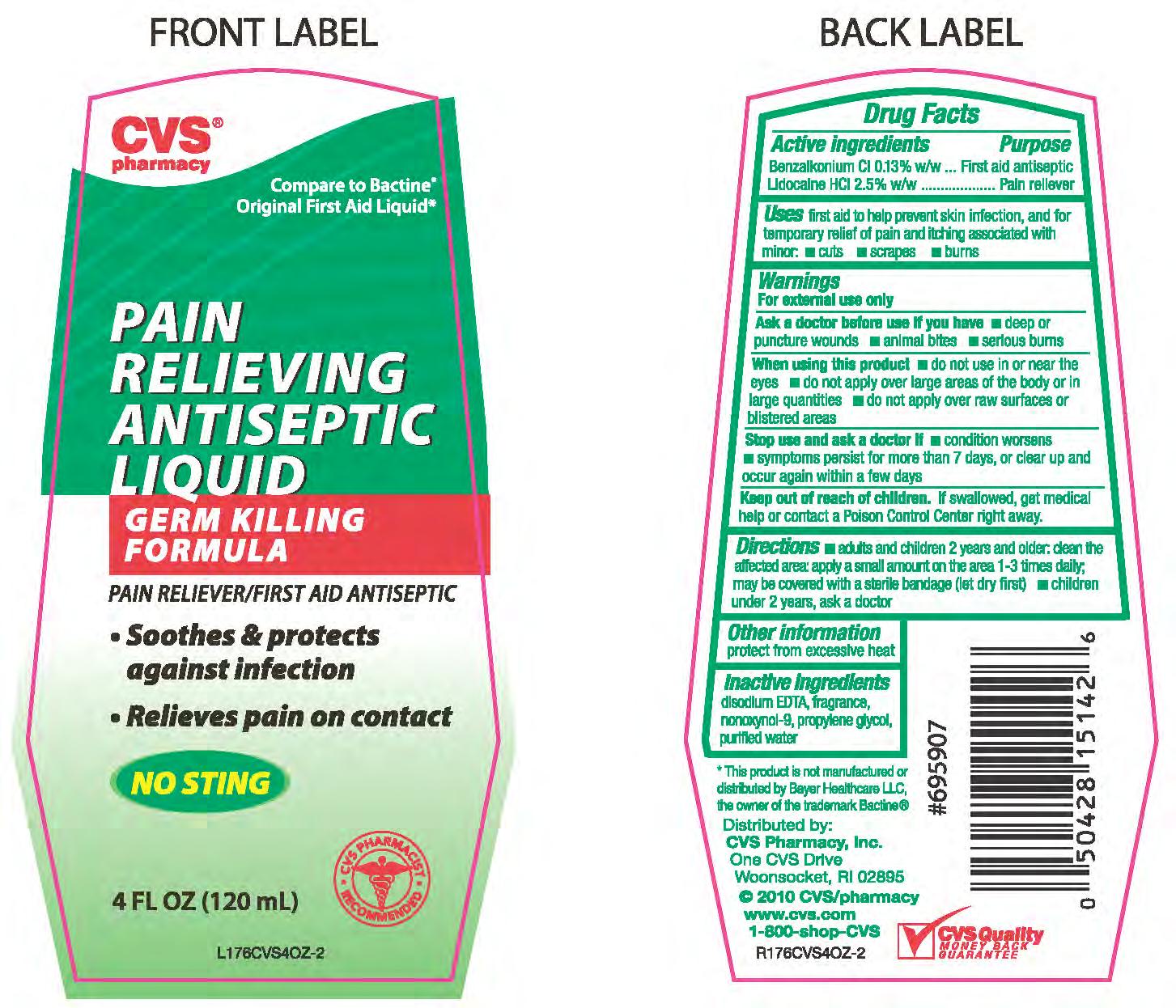
Active ingredient Purpose
Benzalkonium Cl 0.13% w/w...............................Antiseptic
lidocaine HCl 2.5% w/w......................................Pain reliever
Active ingredient Purpose
Benzalkonium Cl 0.13% w/w...............................Antiseptic
lidocaine HCl 2.5% w/w......................................Pain reliever
Uses first aid to help prevent skin infection, and for temporary relief of pain
and itching associated with minor - cuts - scrapes - burns
Keep out of reach of children. If swallowed, get medical help
or contact a Poison Control Center right away.
Directions - adults and children 2 years and older; clean the affected area; apply a
small amount on the area 1-3 times daily; may be covered with a sterile bandage (let dry first)
- children under 2 years, ask a doctor.
Uses first aid to help prevent skin infection, and for temporary relief of pain
and itching associated with minor - cuts - scrapes - burns
Warnings
For external use only.
Ask a doctor before use if you have - deep or puncture wounds
- animal bites - serious burns
When using this product - do not use in or near the eyes - do not
apply over large areas of the body or in large quantities - do not apply
over raw surfaces or blistered areas.
Stop use and ask a doctor if - condition worsens - symptoms
persist for more than 7 days, or clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical
help or contact a Poison Control Center right awayImmediately.