Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- itchy, watery eyes
- •
- sneezing
- •
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
Directions
|
adults and children 6 years and over |
1 tablet daily; not more than 1 tablet in 24 hours |
|
children under 6 years of age |
ask a doctor |
|
consumers with liver or kidney disease |
ask a doctor |
Other information
- •
- do not use if printed foil under cap is broken or missing
- •
- store between 20° to 25°C (68° to 77°F)
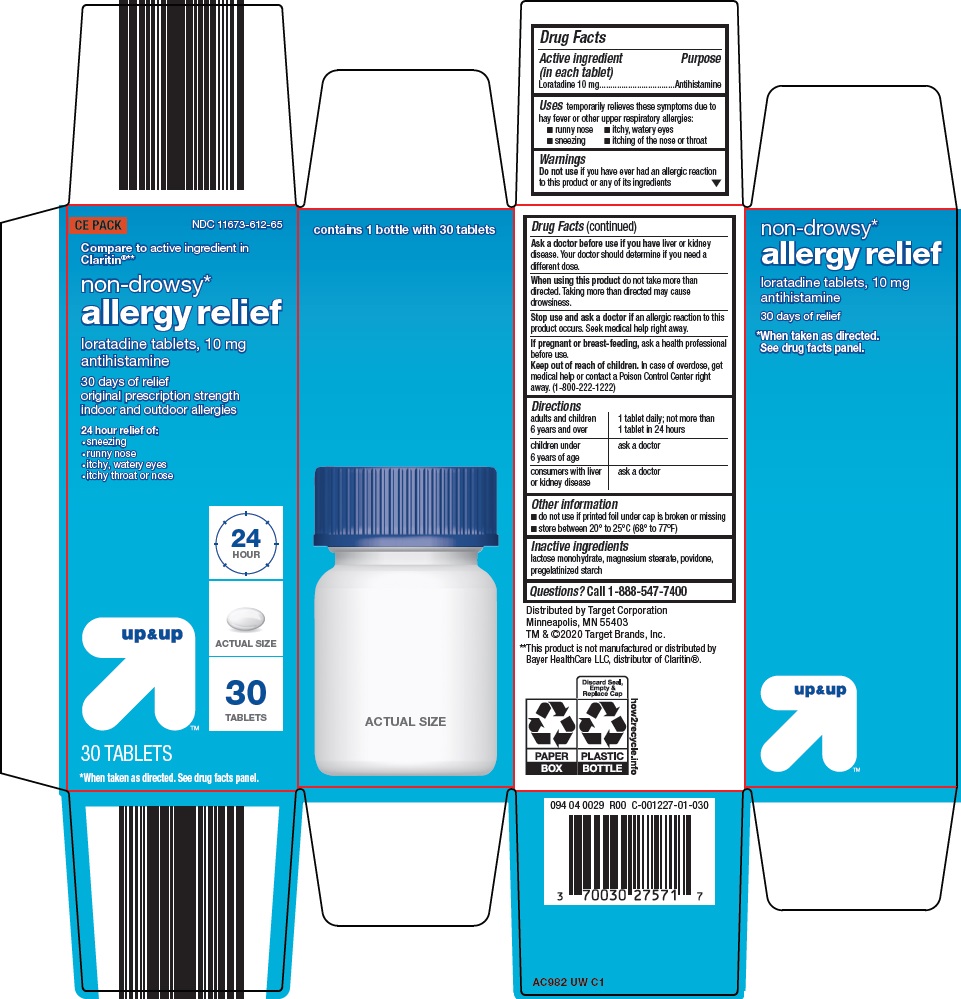
Principal Display Panel
CE PACK
Compare to active ingredient in Claritin®
non-drowsy*
allergy relief
loratadine tablets, 10 mg
antihistamine
30 days of relief
original prescription strength
indoor and outdoor allergies
24 hour relief of:
sneezing
runny nose
itchy, watery eyes
itchy throat or nose
24 HOUR
ACTUAL SIZE
30 TABLETS
30 TABLETS
*When taken as directed. See drug facts panel.