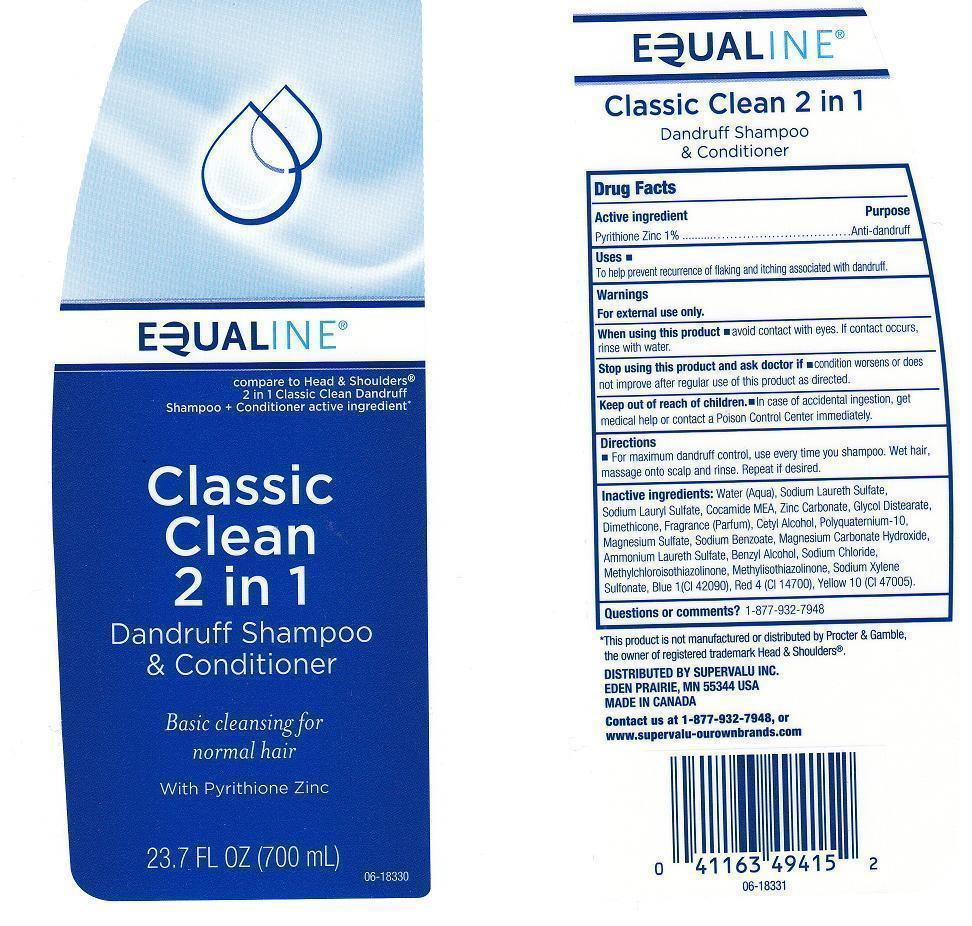
EQUALINE CLASSIC CLEAN 2 IN 1- pyrithione zinc liquid
SUPERVALU INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT
PYRITHIONE ZINC 1%
USES
TO HELP PREVENT RECURRENCE OF FLAKING AND ITCHING ASSOCIATED WITH DANDRUFF.
WARNINGS
FOR EXTERNAL USE ONLY.
WHEN USING THIS PRODUCT
AVOID CONTACT WITH EYES. IF CONTACT OCCURS, RINSE WITH WATER.
STOP USING THIS PRODUCT AND ASK DOCTOR IF
CONDITION WORSENS OR DOES NOT IMPROVE AFTER REGULAR USE OF THIS PRODUCT AS DIRECTED.
KEEP OUT OF REACH OF CHILDREN
IN CASE OF ACCIDENTAL INGESTION, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
DIRECTIONS
FOR MAXIMUM DANDRUFF CONTROL, USE EVERY TIME YOU SHAMPOO. WET HAIR, MASSAGE ONTO SCALP AND RINSE. REPEAT IF DESIRED.
INACTIVE INGREDIENTS:
WATER (AQUA), SODIUM LAURETH SULFATE, SODIUM LAURYL SULFATE, COCAMIDE MEA, ZINC CARBONATE, GLYCOL DISTEARATE, DIMETHICONE, FRAGRANCE (PARFUM), CETYL ALCOHOL, POLYQUATERNIUM-10, MAGNESIUM SULFATE, SODIUM BENZOATE, MAGNESIUM CARBONATE HYDROXIDE, AMMONIUM LAURETH SULFATE, BENZYL ALCOHOL, SODIUM CHLORIDE, METHYLCHLOROISOTHIAZOLINONE, METHYLISOTHIAZOLINONE, SODIUM XYLENE SULFONATE, BLUE 1 (CI 42090), RED 4 (CI 14700), YELLOW 10 (CI 47005).
QUESTIONS OR COMMENTS?
1-877-932-7948
LABEL COPY
SUPERVALU INC.
