TINACTIN- tolnaftate cream
Bayer HealthCare LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Tolnaftate 1%
Uses
- proven clinically effective in the treatment of most athlete's foot (tinea pedis) and ringworm (tinea corporis)
- helps prevent most athlete's foot with daily use
- for effective relief of itching, burning and cracking
Warnings
For external use only
Do not use on children under 2 years of age except under the advice and supervision of a doctor.
When using this product avoid contact with the eyes
Stop use and ask a doctor if
- irritation occurs
- there is no improvement within 4 weeks
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wash affected area and dry thoroughly
- apply a thin layer over affected area twice daily (morning and night)
- supervise children in the use of this product
- for athlete's foot: pay special attention to spaces between the toes; wear well-fitting, ventilated shoes and change shoes and socks at least once daily
- use daily for 4 weeks; if condition persists longer, ask a doctor
- to prevent athlete's foot, apply once or twice daily (morning and/or night)
- this product is not effective on the scalp or nails
Other information
store between 20° to 25°C (68° to 77°F)
Inactive ingredients
ceteth-20, cetostearyl alcohol, chlorocresol, mineral oil, propylene glycol, purified water, sodium phosphate monobasic, white petrolatum
Questions?
1-866-360-3266
Distributed by
Bayer HealthCare LLC, Whippany, NJ, USA, 07981
PRINCIPAL DISPLAY PANEL - 15 g Tube Carton
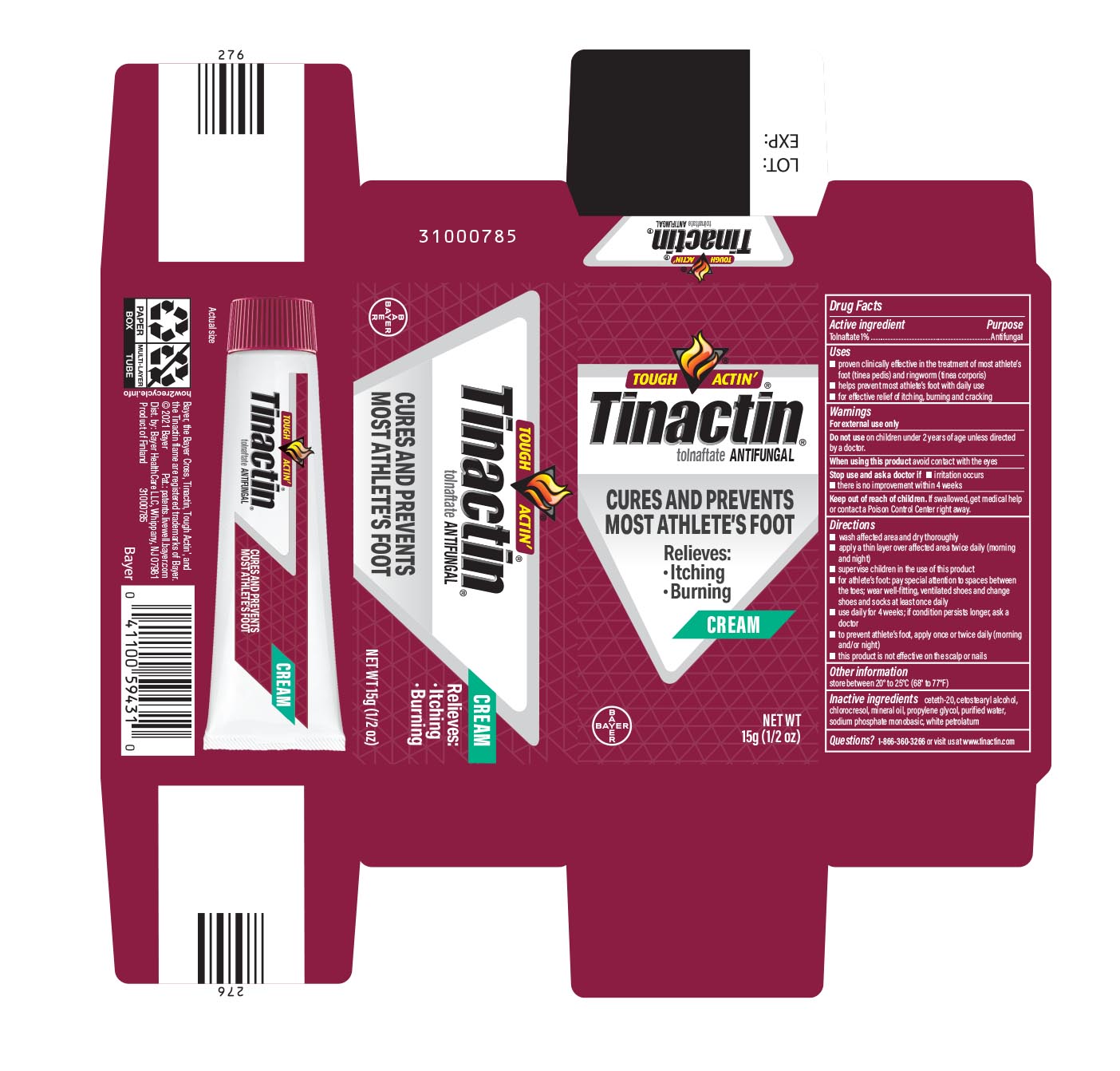
TOUCH ACTIN'
Tinactin
®
tolnaftate
ANTIFUNGAL
CURES AND PREVENTS
MOST ATHLETE'S FOOT
Relieves:
CREAM
NET WT 15G (1/2 OZ)
Bayer HealthCare LLC.
