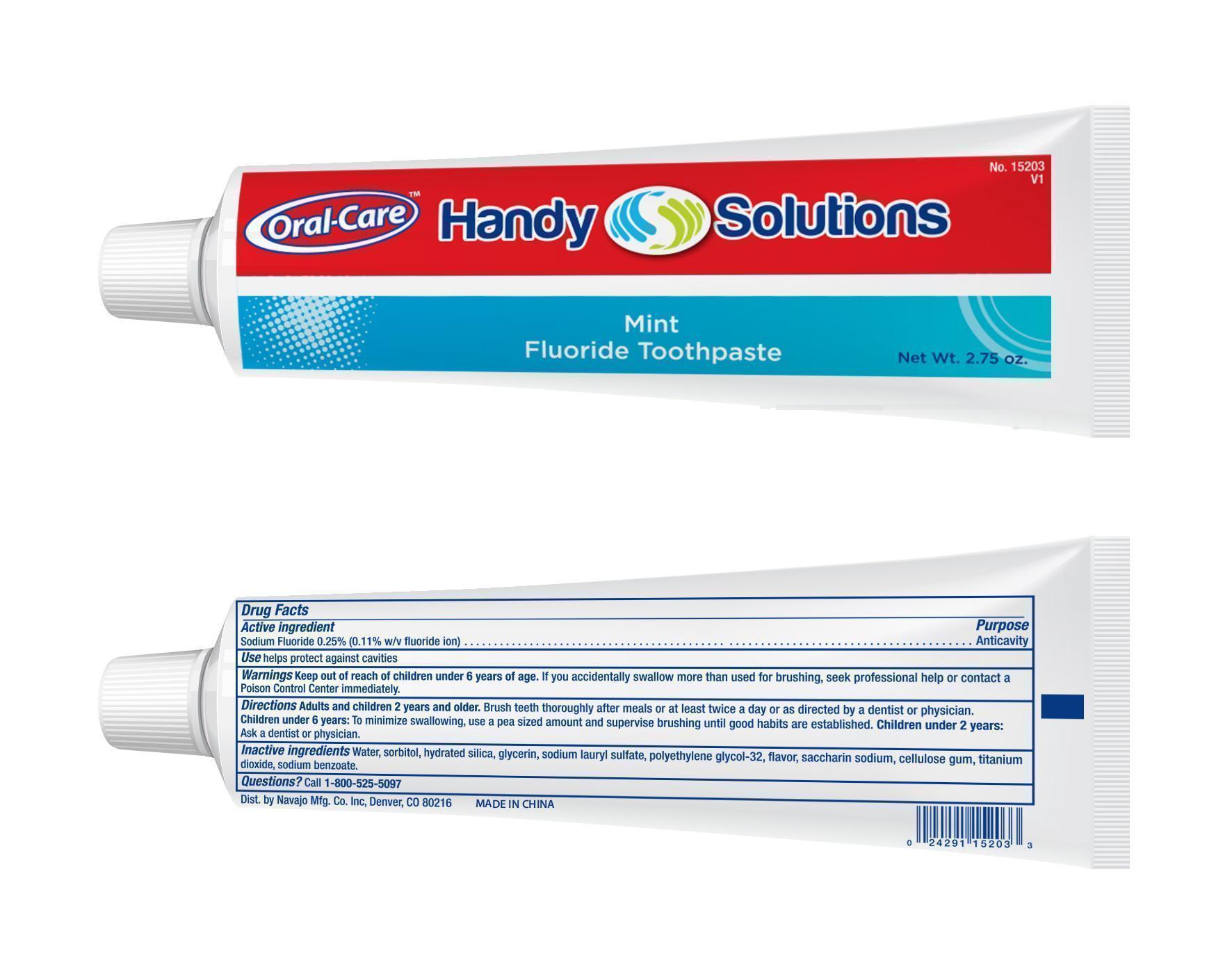
HANDY SOLUTIONS MINT FLUORIDE TOOTH- sodium fluoride paste
Navajo Manufacturing Co., Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Sodium Fluoride 0.25%
Use
helps protect against cavities
Warnings
Keep out of reach of children under 6 years of age.
If you accidentally swallow more than used for brushing, seek professional help or contact a Poison Control Center Immediately
Directions
Adults and children 2 years and older. Brush teeth thoroughly after meals or at least twice a day or as directed by a dentist or physician. Children under 6 years: To minimize swallowing, use a pea sized amount and supervise brushing until good habits are established. Children under 2 years: Ask a dentist or physician
Inactive ingredients
Water, Sorbitol, Hydrated Silica, Glycerin, Sodium Lauryl Sulfate, Polyethylene Glycol-32, Flavor, Saccharin Sodium, Cellulose Gum, Titanium Dioxide, Sodium Benzoate.
Questions?
Call 1-800-525-5097
Product Label
Navajo Manufacturing Co., Inc.
