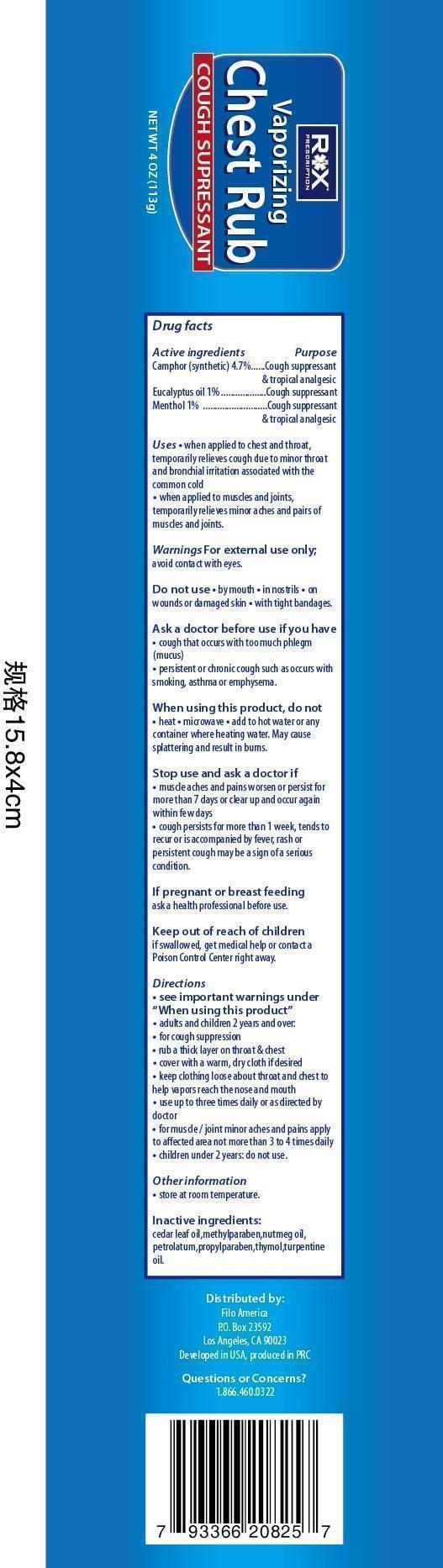
Use
when applied to chest and throat
- temporarily relieves cough due to minor throat and bronchial irritation associated with a cold
when applied to muscles and joints
- temporarily relieves minor aches and pains of muscles and joints
Warnings
For external use only; avoid contact with eyes.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
When using this product
- do not heat
- do not microwave
- do not add to hot water or any container where heating water. May cause splattering and result in burns.
- do not take by mouth or place in nostrils
Directions
- see important warnings under "When using this product"
- adults and children 2 year and over.
for cough suppression
- rub a thick layer on the throat and chest
- cover with a warm, dry cloth if desired
- keep clothing loose about throat and chest to help vapors reach the nose and mouth
- use up to 3 times daily, or as directed by a doctor
for muscle/joint minor aches and pains apply
- to affected area not more than 3-4 times daily
- children under 2 yeas : ask a doctor
other information
- store at room temperature
if pregnant or breast feeding
- ask a health professional before use