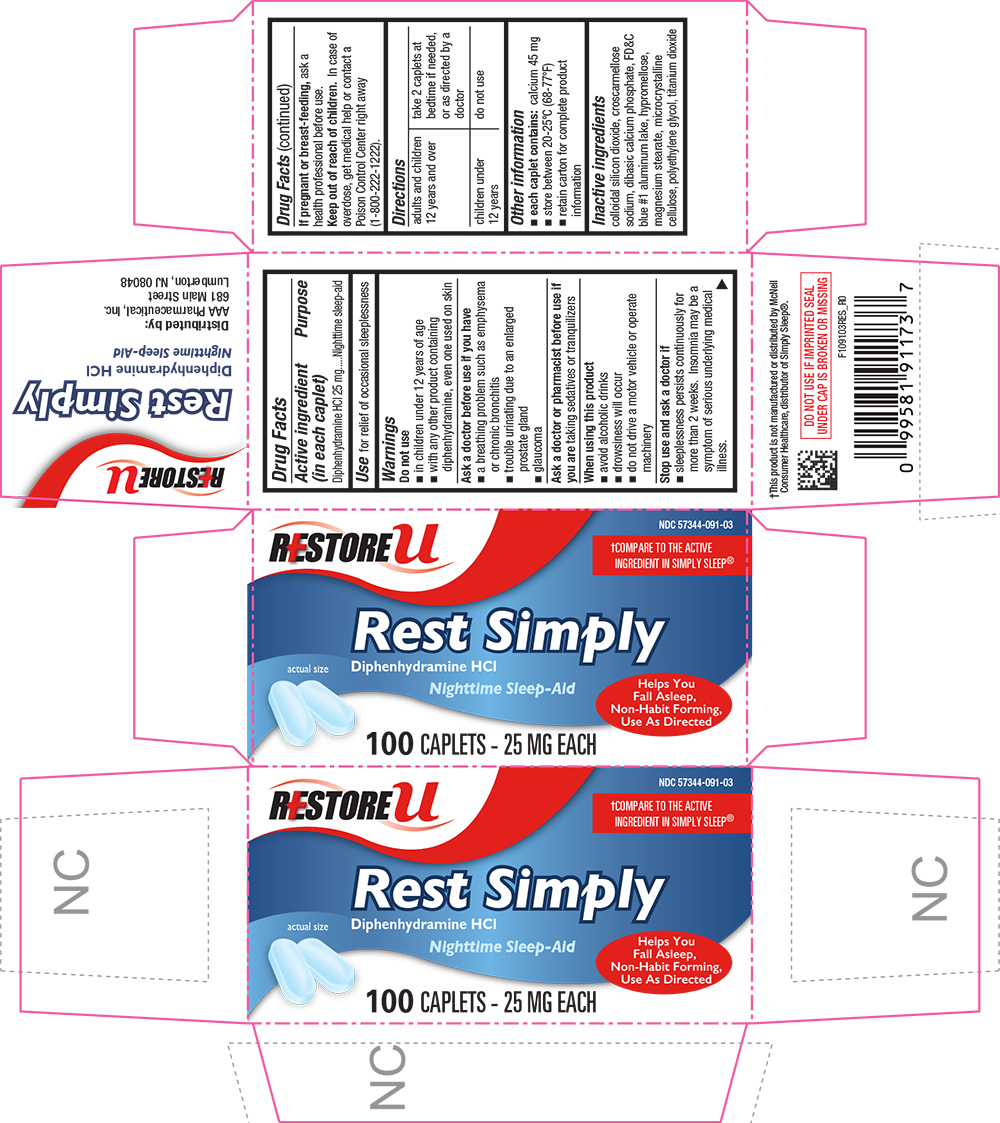
DIPHENHYDRAMINE HYDROCHLORIDE- diphenhydramine hydrochloride tablet, coated
AAA Pharmaceutical, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each caplet)
Diphenhydramine HCl 25 mg
Purpose
Nighttime sleep-aid
Use
for relief of occasional sleeplessness
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on skin
- in children under 12 years of age
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- glaucoma
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
When using this product avoid alcoholic drinks
Stop use and ask a doctor if sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
| adults and children 12 years of age and over | take 2 caplets at bedtime if needed, or as directed by a doctor |
| children under 12 years of age | do not use |
Other information
- each caplet contains:
calcium 45 mg
- store at room temperature 15°-30°C (59°-86°F)
- retain carton for complete product information
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, dibasic calcium phosphate, FD&C blue #1, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, titanium dioxide
Distributed by:
AAA Pharmaceutical, Inc.
681 Main Street
Lumberton, NJ 08048
PRINCIPAL DISPLAY PANEL - 24 Caplet Carton
RESTORE U
NDC 57344-091-03
†COMPARE TO THE ACTIVE INGREDIENT IN SIMPLY SLEEP
®
Rest Simply
actual size
Diphehydramine HCl
Nighttime Sleep-Aid
Helps You Fall Asleep Fast, Non-Habit Forming, Use as Directed
100 CAPLETS - 25 MG EACH
AAA Pharmaceutical, Inc.
