Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- the intensity of coughing
- the impulse to cough to help you get to sleep
Warnings
Do not use
- for children under 12 years of age
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Directions
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for timing of meals
- adults and children 12 years and older: 1 or 2 tablets every 12 hours; not more than 4 tablets in 24 hours
- children under 12 years of age: do not use
Inactive ingredients
carbomer homopolymer type B; D&C yellow #10 aluminum lake; hypromellose, USP; magnesium stearate, NF; microcrystalline cellulose, NF; sodium starch glycolate, NF
Product manufactured by:
Reckitt Benckiser, Parsippany, NJ 07054-0224
Repackaged and distributed with permission of manufacturer by:
Lil' Drug Store Products, Inc., 1201 Continental Place NE, Cedar Rapids, IA 52402
Mucinex ® DM 2ct, Lil' Drug Store ® - PDP/Package
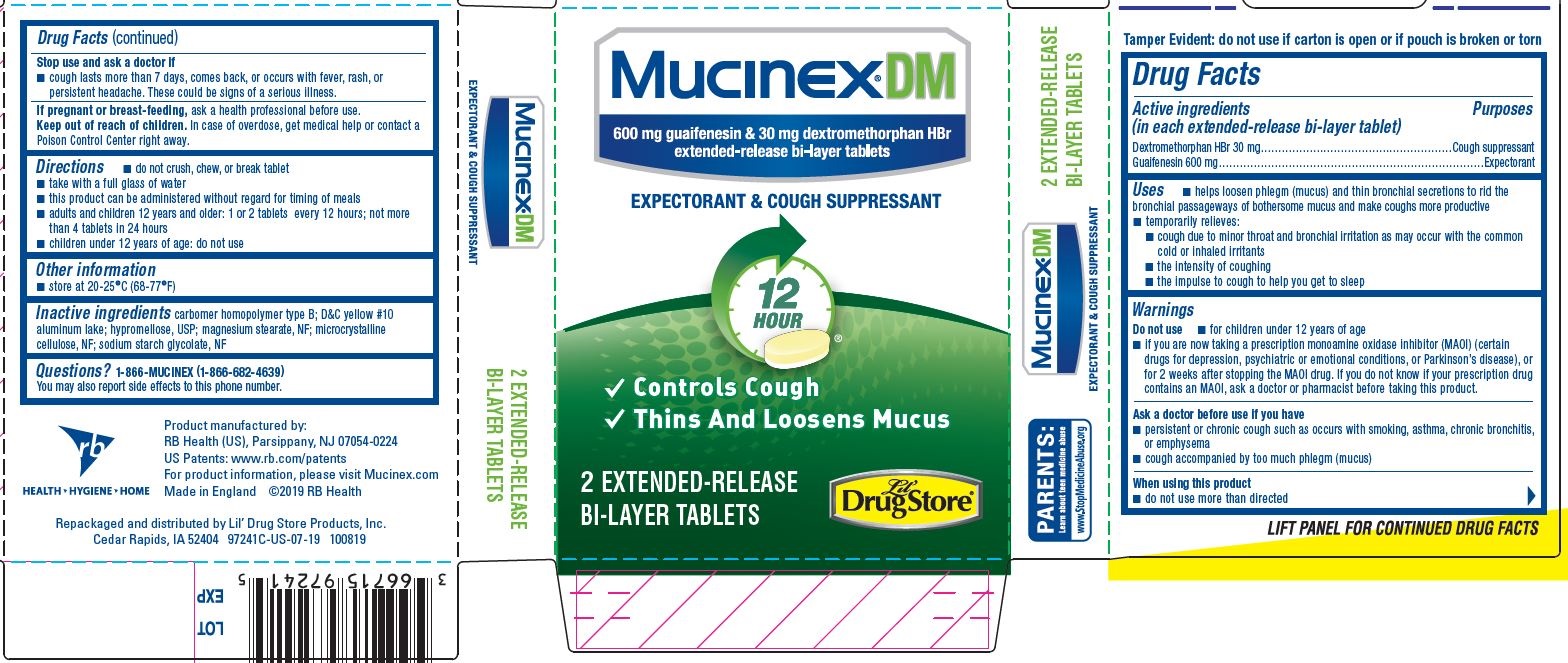
Mucinex® DM
600 mg guaifenesin & 30 mg dextromethorphan HBr
extended-release bi-layer tablets
EXPECTORANT & COUGH SUPPRESSANT
12 HOUR
[caplet image]
- Controls Cough
- Thins And Loosens Mucus
2 EXTENDED-RELEASE BI-LAYER TABLETS
[Lil' Drug Store® logo]
Mucinex ® DM 2ct, TRAVEL BASIX - PDP/Package
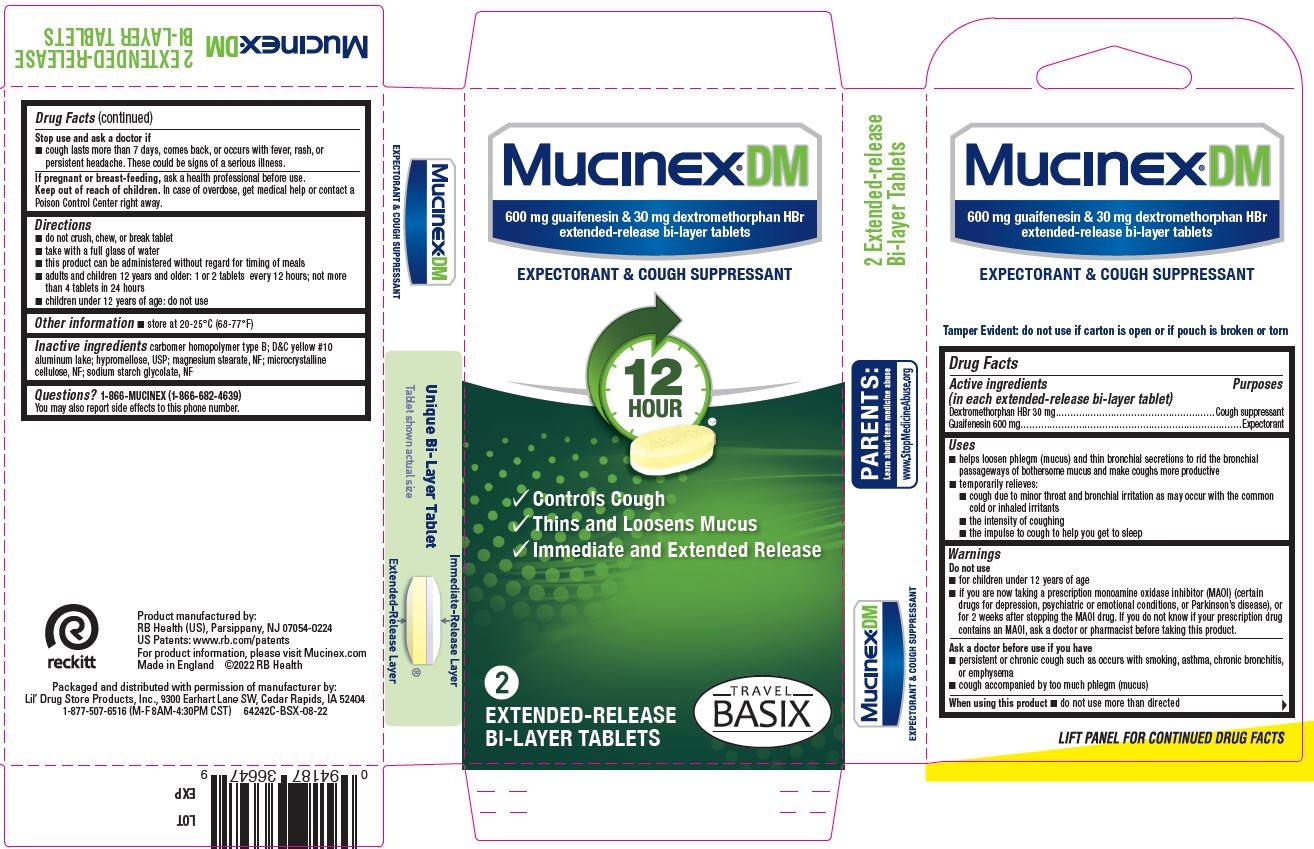
Mucinex ® DM
600 mg guaifenesin & 30 mg dextromethorphan HBr
extended-release bi-layer tablets
EXPEXTORANT & COUGH SUPPRESSANT
12
HOUR
[tablet image]
[check mark] Controls Cough
[check mark] Thins and Loosens Mucus
[check mark] Immediate and Extended release
2
EXTENDED-RELEASE
BI-LAYER TABLETS
[TRAVEL BASIX logo]
 ®
®
Mucinex ® DM 2ct CVP ® HEALTH - PDPPackage
Mucinex ® DM
600 mg guaifenesin & 30 mg dextromethorphan HBr
extended-release bi-layer tablets
EXPEXTORANT & COUGH SUPPRESSANT
12
HOUR
[tablet image]
[check mark] Controls Cough
[check mark] Thins and Loosens Mucus
[check mark] Immediate and Extended release
2
EXTENDED-RELEASE
BI-LAYER TABLETS
[CVP ® HEALTH logo]
