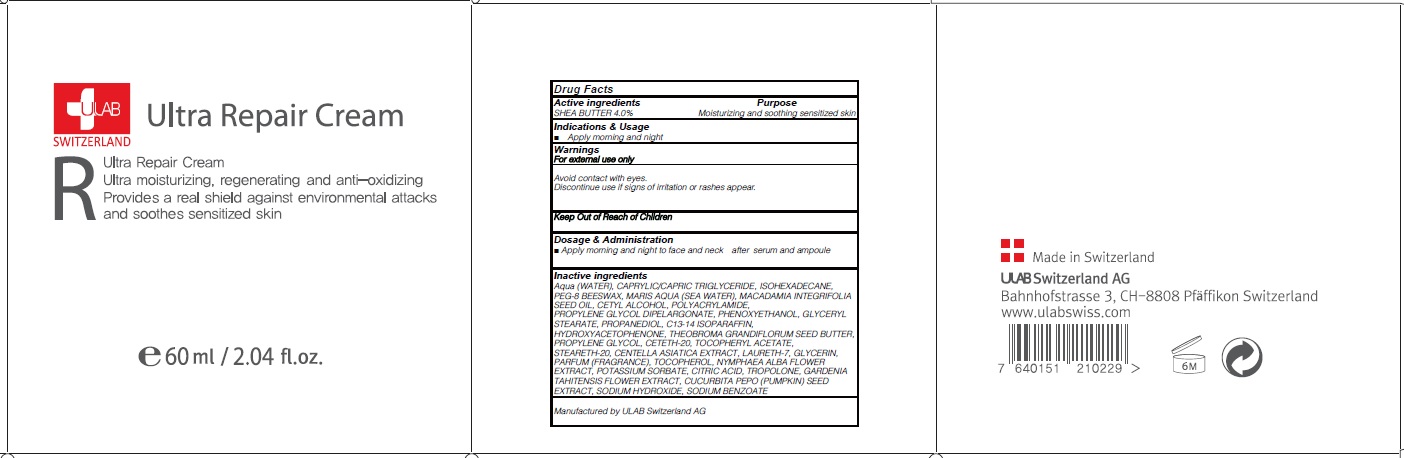
INACTIVE INGREDIENT
Inactive ingredients: Aqua (WATER), CAPRYLIC/CAPRIC TRIGLYCERIDE, ISOHEXADECANE, PEG-8 BEESWAX, MARIS AQUA (SEA WATER), MACADAMIA INTEGRIFOLIA SEED OIL, CETYL ALCOHOL, POLYACRYLAMIDE, PROPYLENE GLYCOL DIPELARGONATE, PHENOXYETHANOL, GLYCERYL STEARATE, PROPANEDIOL, C13-14 ISOPARAFFIN, HYDROXYACETOPHENONE, THEOBROMA GRANDIFLORUM SEED BUTTER, PROPYLENE GLYCOL, CETETH-20, TOCOPHERYL ACETATE, STEARETH-20, CENTELLA ASIATICA EXTRACT, LAURETH-7, GLYCERIN, PARFUM (FRAGRANCE), TOCOPHEROL, NYMPHAEA ALBA FLOWER EXTRACT, POTASSIUM SORBATE, CITRIC ACID, TROPOLONE, GARDENIA TAHITENSIS FLOWER EXTRACT, CUCURBITA PEPO (PUMPKIN) SEED EXTRACT, SODIUM HYDROXIDE, SODIUM BENZOATE
WARNINGS
Warnings: For external use only Avoid contact with eyes. Discontinue use if signs of irritation or rashes appear.