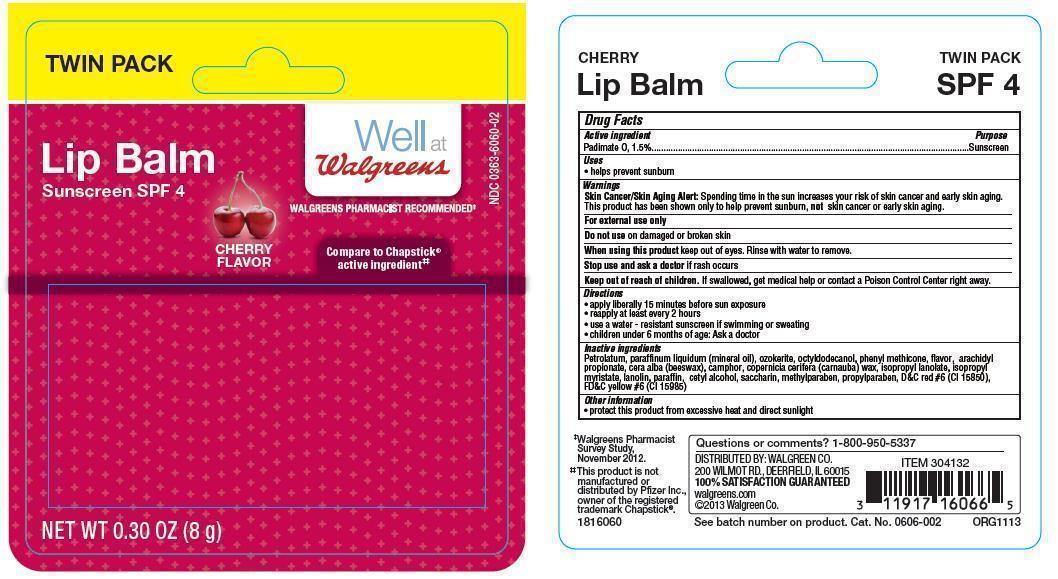
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
For external use only
Directions
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water-resistant sunscreen if swimming or sweating
- children under 6 months of age: Ask a doctor
Inactive ingredients
Petrolatum, paraffinum liquidum (mineral oil), ozokerite, octyldodecanol, phenyl methicone, flavor, arachidyl propionate, cera alba (beeswax), camphor, copernicia cerifera (carnauba) wax, isopropyl lanolate, isopropyl myristate, lanolin, paraffin, cetyl alcohol, saccharin, methylparaben, propylparaben, D&C red #6 (CI 15850), FD&C yellow #6 (CI 15985)