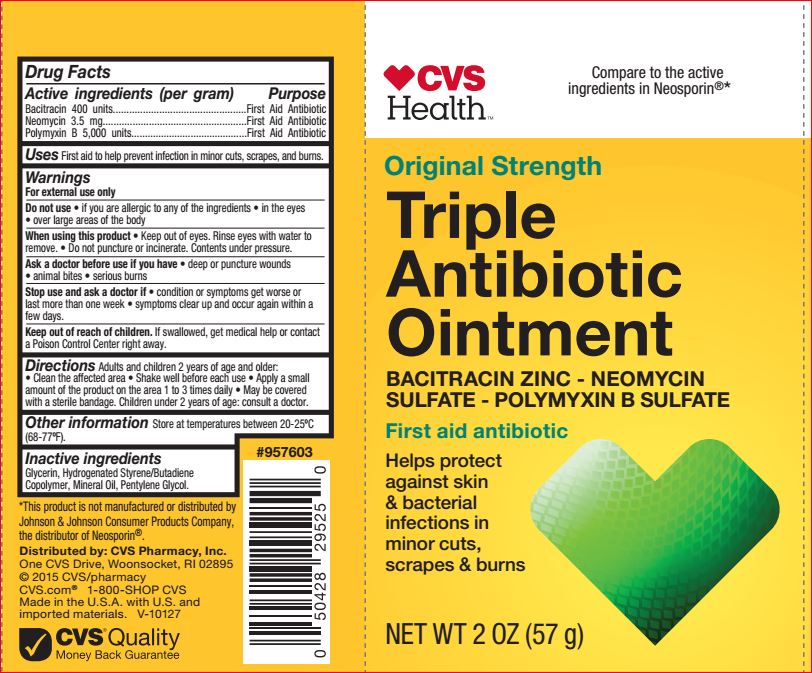
Uses
First aid to help prevent infection and for temporary relief of pain or discomfort in minor: cuts, scrapes, and burns.
Warnings
For external use only. Do not use in the eyes, over large areas of the body. When using this product keep out of eyes. Rinse eyes with water to remove. Do not puncture or incinerate. Contents under pressure. Ask a doctor before use if you have condition or symptoms get worse or last more than one week, symptoms clear up and occur again within a few days.
Directions
Adults and children 2 years of age and older: clean the affected area, shake well before each use, spray a small amount of the product on the area 1 to 3 times daily. May be covered with a sterile bandage. Children under 2 years of age: consult a doctor.
Inactive ingredients
Glycerin, Hydrogenated Styrene/Butadiene Copolymer, Mineral Oil, Pentylene Glycol