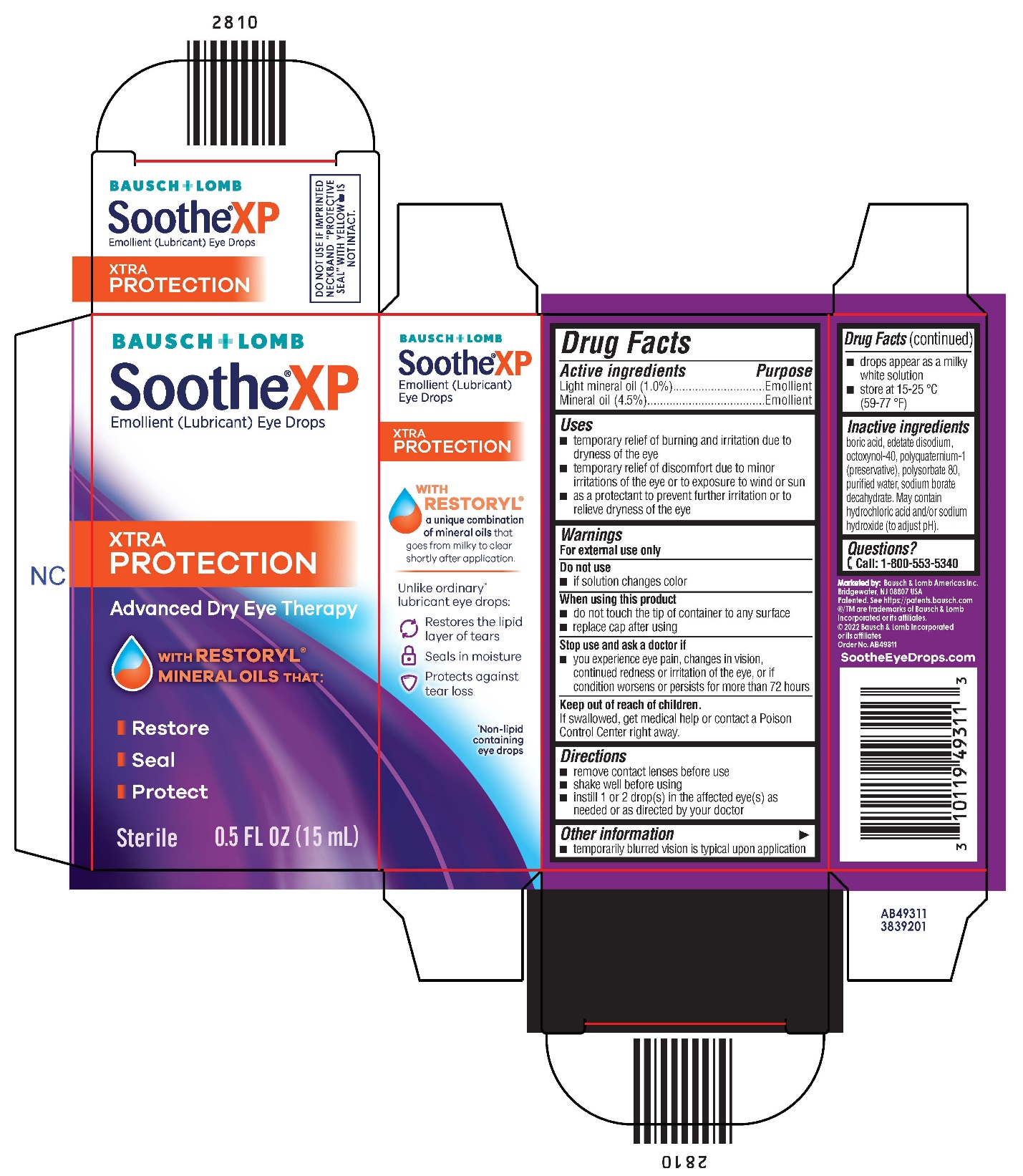
Uses
- •
- temporary relief of burning and irritation due to dryness of the eye
- •
- temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
- •
- as a protectant to prevent further irritation or to relieve dryness of the eye
Warnings
For external use only
When using this product
- •
- do not touch the tip of container to any surface
- •
- replace cap after using
Directions
- •
- remove contact lenses before use
- •
- shake well before using
- •
- instill 1 or 2 drop(s) in the affected eye(s) as needed or as directed by your doctor
Other information
- •
- temporarily blurred vision is typical upon application
- •
- drops appear as a milky white solution
- •
- store at 15-25 °C (59-77 °F)
Inactive ingredients
boric acid, edetate disodium, octoxynol-40, polyquaternium-1 (preservative), polysorbate 80, purified water, sodium borate decahydrate. May contain hydrochloric acid and/or sodium hydroxide (to adjust pH).
Questions?
[phone icon] Call: 1-800-553-5340
Marketed by: Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Patented. See https://patents.bausch.com
®/TM are trademarks of Bausch & Lomb
Incorporated or its affiliates.
© 2022 Bausch & Lomb Incorporated
or its affiliates
Order No. AB49311
SootheEyeDrops.com