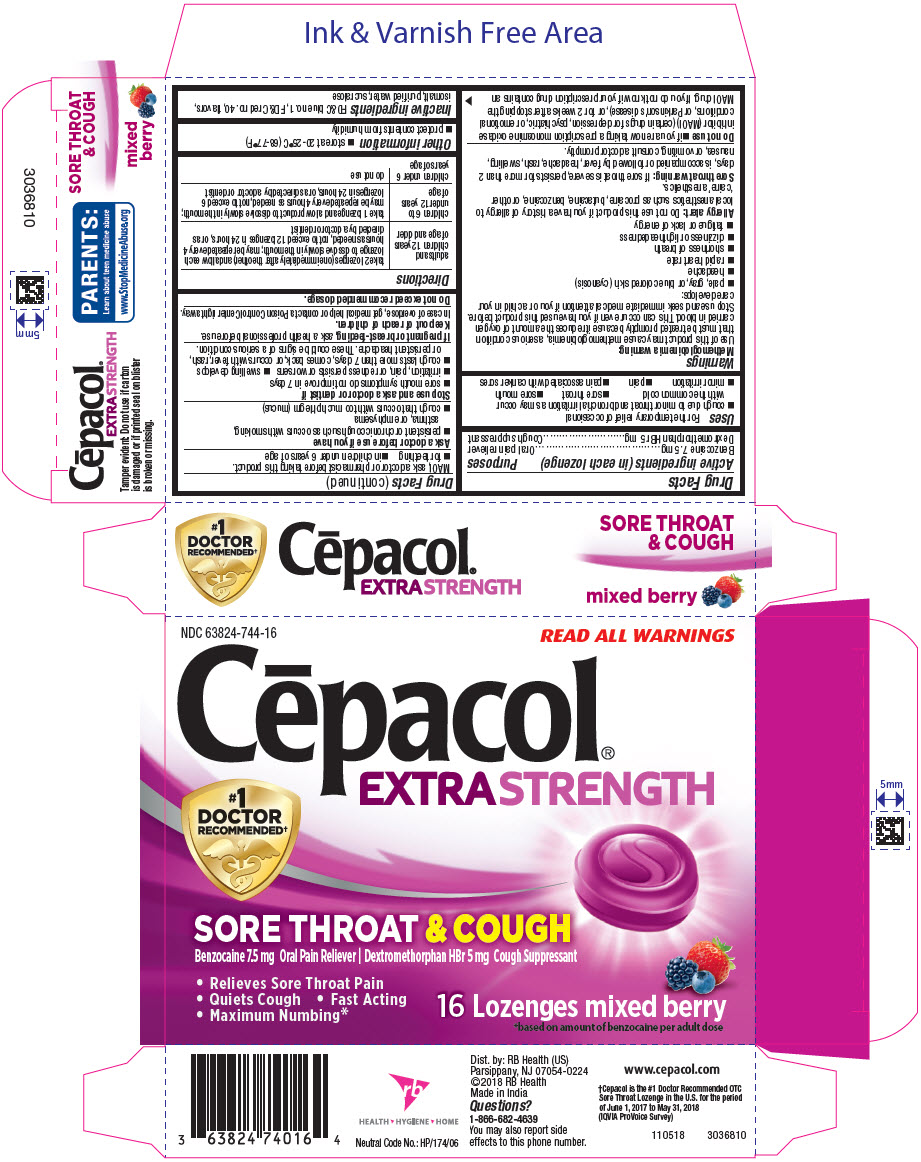
Uses
For the temporary relief of occasional
- cough due to minor throat and bronchial irritation as may occur with the common cold
- sore throat
- sore mouth
- minor irritation
- pain
- pain associated with canker sores
Warnings
Methemoglobinemia warning
Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray, or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Allergy alert
Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other 'caine' anesthetics.
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea, or vomiting, consult a doctor promptly.
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- for teething
- in children under 6 years of age
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- cough that occurs with too much phlegm (mucus)
Stop use and ask a doctor or dentist if
- sore mouth symptoms do not improve in 7 days
- irritation, pain, or redness persists or worsens
- swelling develops
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious condition.
Directions
| adults and children 12 years of age and older | take 2 lozenges (one immediately after the other) and allow each lozenge to dissolve slowly in the mouth; may be repeated every 4 hours as needed, not to exceed 12 lozenges in 24 hours, or as directed by a doctor or dentist |
| children 6 to under 12 years of age | take 1 lozenge and allow product to dissolve slowly in the mouth; may be repeated every 4 hours as needed, not to exceed 6 lozenges in 24 hours, or as directed by a doctor or dentist |
| children under 6 years of age | do not use |
PRINCIPAL DISPLAY PANEL - 16 Lozenge Blister Pack Carton
NDC 63824-744-16
READ ALL WARNINGS
Cēpacol
®
EXTRA STRENGTH
#1
DOCTOR
RECOMMENDED
†
SORE THROAT & COUGH
Benzocaine 7.5 mg Oral Pain Reliever | Dextromethorphan HBr 5 mg Cough Suppressant
- Relieves Sore Throat Pain
- Quiets Cough • Fast Acting
- Maximum Numbing*
16 Lozenges mixed berry
*based on amount of benzocaine per adult dose