Keep out of reach of children. In case of overdose, get medical help right away and contact a poison control center right away.
Adults and children 12 years and over: Take 2 tablets at bedtime(Do not take more than 2 tablets in a 24 hour time)
Do not use this product if you have had an allergic to Acetaminophen or any of the inactive ingredients. Ask a doctor before taking this product if you have Liver disease or are taking blood thinning medication. Do not take more than directed.If pregnant or breast feeding contact a doctor before using this product. Stop use and consult a doctor if: Pain gets worse or last more than 10 days: If fever gets worse or last more than 3 days: new symptoms appear: redness or swelling appear.
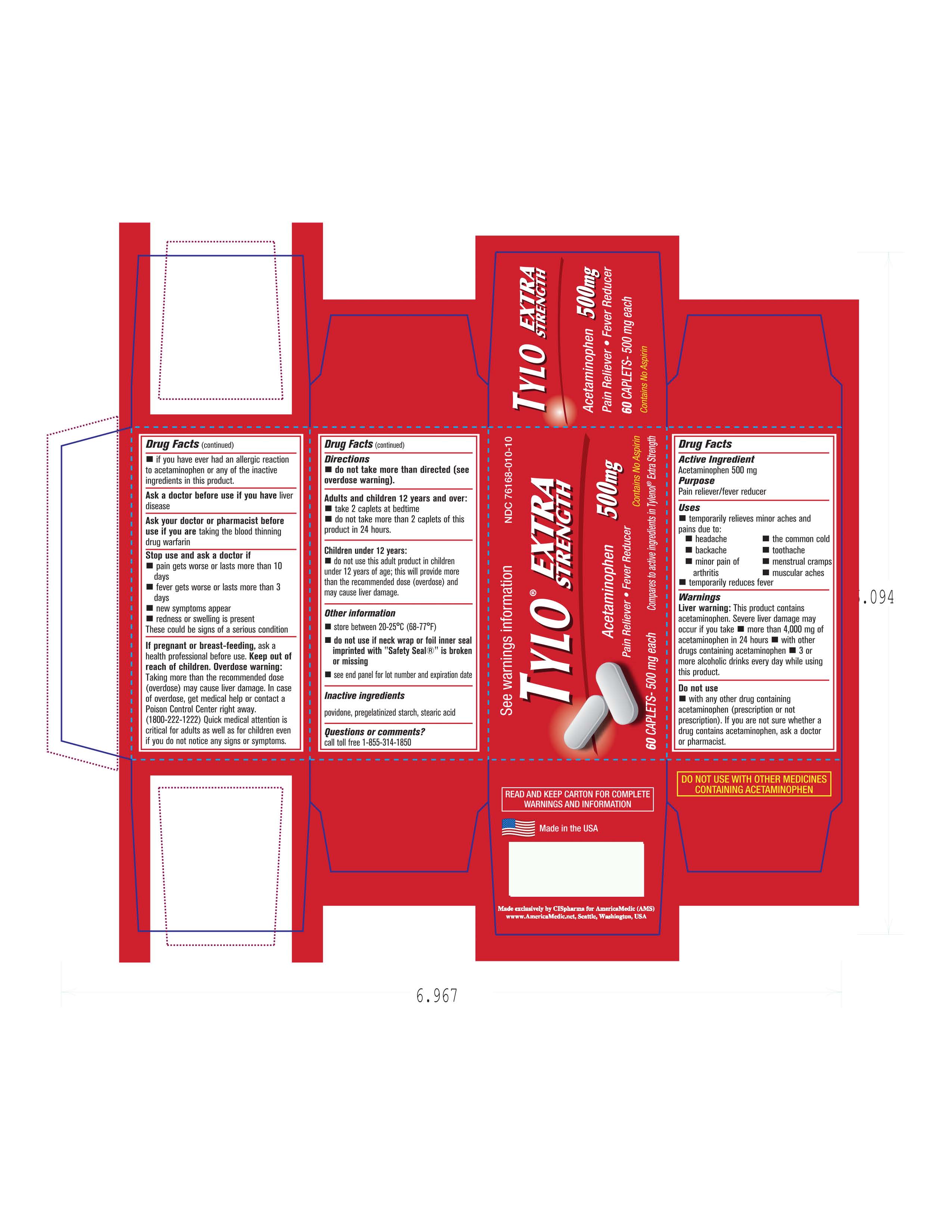 image of carton
image of carton