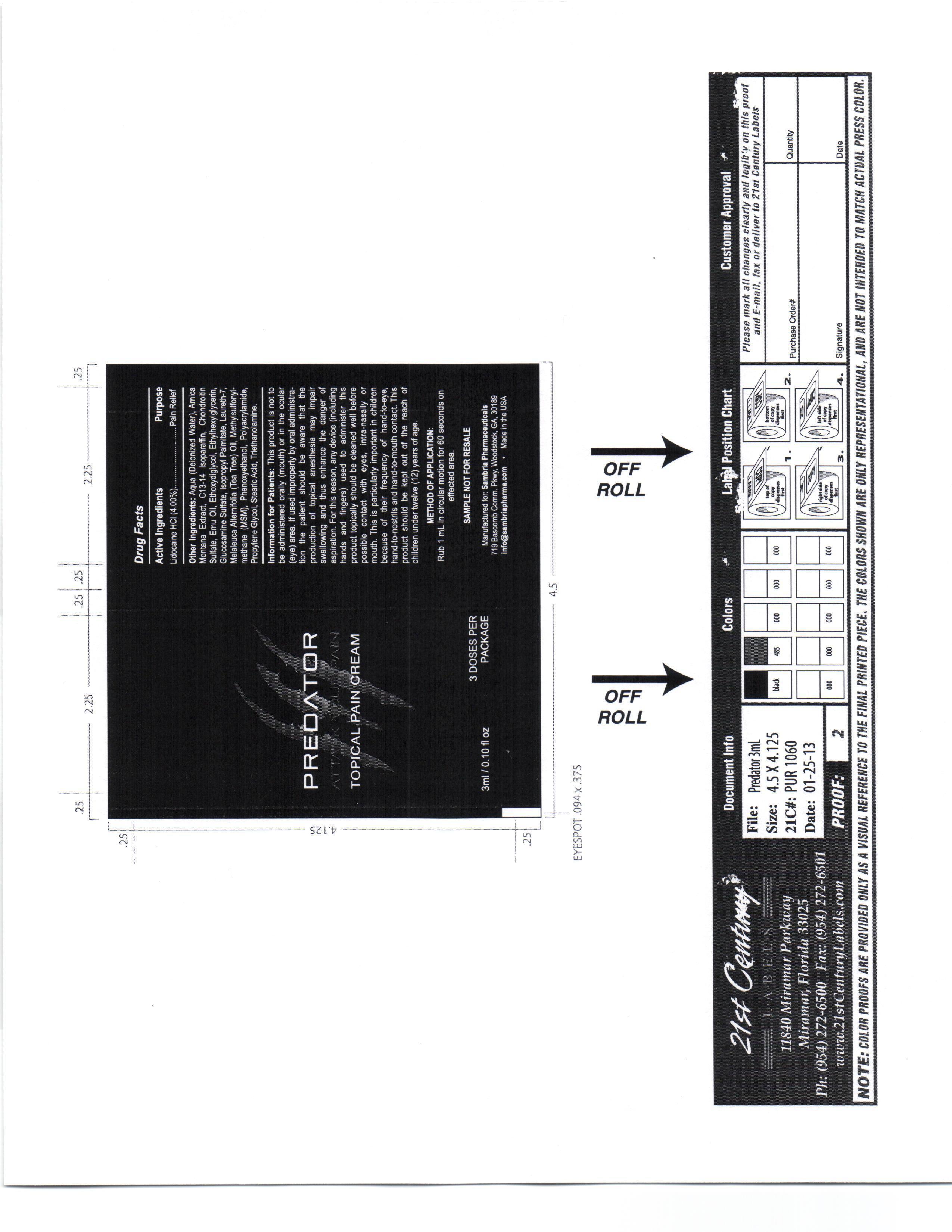
Information for Patients
This product is not to be administered orally (mouth) or in the ocular (eye) area.
If used improperly by oral administration the patient should be aware that the production of topical anesthesia may impair swallowing and thus enhance the danger of aspiration. For this reason, any device (including hands and fingers) used to administer this product topically should be cleaned well before possible contact with eyes, intra-nasaly or mouth.
Other ingredients
Aqua, Amica Montana Extract, C13-14 Isoparafin, Chondrotin Sulfate, Emu Oil, Ethoxydiglycol, Ethylhexylglycerin, Glucosamine Sulfate, Isopropyl Palmitate, Laureth 7, Melaleuca Alternifoil (Tea Tree) oil, Methylsulfonylmethana (MSM), Phenoxyethanol, Polyacrylamide, Propylene Glycol, Stearic Acid, Triethanolamine