VanaLice Lice Killing Gel Lice Treatment - piperonyl butoxide, pyrethrum extract
8 FL OZ.
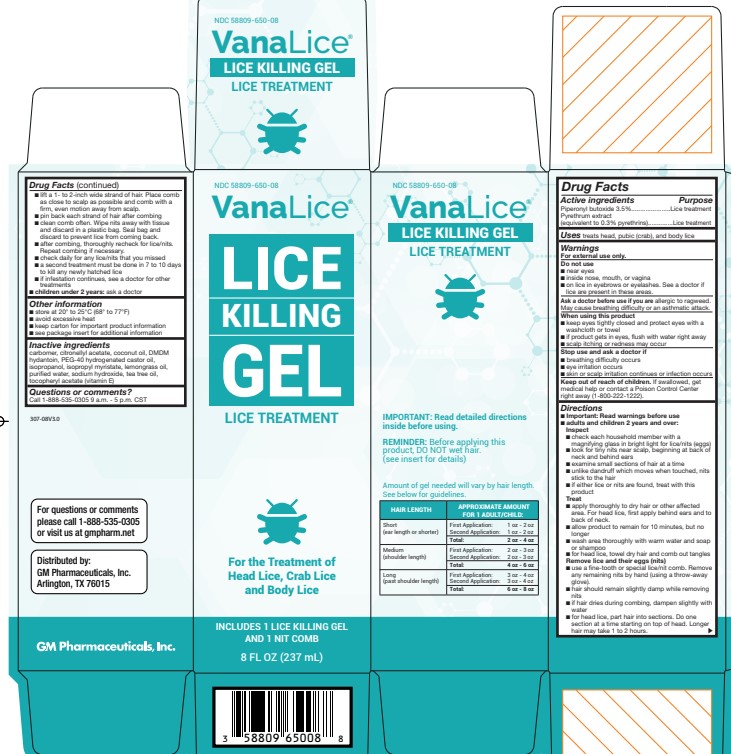
VanaLice Lice Killing Gel Lice Treatment Drug Facts
Warnings
For external use only
Do not use
- near eyes
- inside nose, mouth, or vagina
- on lice in eyebrows or eyelashes. See a doctor if lice are present in these areas.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you are
- allergic to ragweed. May cause breathing difficulty or an asthmatic attack.
When using this product
- keep eyes tightly closed and protect eyes with a washcloth or towel
- if product gets into eyes, flush with water right away
- scalp itching or redness may occur
Directions
- Important: Read warnings before use Adults and children 2 years and over:
Inspect
- check each household member with a magnifying glass in bright light for lice/nits (eggs)
- look for tiny nits near scalp, beginning at back of neck and behind ears
- examine small sections of hair at a time
- unlike dandruff which moves when touched, nits stick to the hair
- if either lice or nits are found, treat with this product
Treat
- apply thoroughly to DRY HAIR or other affected area. For head lice, first apply behind ears and to back of neck.
- allow product to remain for 10 minutes, but no longer
- wash area thoroughly with warm water and soap or shampoo
- for head lice, towel dry hair and comb out tangles
Remove lice and their eggs (nits)
- use a fine-tooth or special lice/nit comb. Remove any remaining nits by hand (using a throw-away glove).
- hair should remain slightly damp while removing nits
- if hair dries during combing, dampen slightly with water
- for head lice, part hair into sections. Do one section at a time starting on top of the head. Longer hair may take 1 to 2 hours.
- lift a 1-to 2-inch wide strand of hair. Place comb as close to scalp as possible and comb with a firm, even motion away from scalp.
- pin back each strand of hair after combing
- clean comb often. Wipe nits away with tissue and discard in a plastic bag. Seal bag and discard to prevent lice from coming back.
- after combing, thoroughly recheck for lice/nits. Repeat combing if necessary.
- check daily for any lice/nits that you missed
- a second treatment must be done in 7 to 10 days to kill any newly hatched lice
- if infestation continues, see a doctor for other treatments
Children under 2 years: ask a doctor
Other information
- store at 20°-25°C (68°-77°F)
- avoid excessive heat
- keep carton for important product information
- see Consumer Information Insert for additional information
Inactive ingredients
carbomer, citronellyl acetate, coconut oil, DMDM hydantoin, peg40-hydrogenated castor oil, isopropanol, isopropyl myristate, lemongrass oil, purified water, sodium hydroxide, tea tree oil tocopheryl acetate (vitamin E)
Questions or comments?
1-888-535-0305 Monday-Friday 9 AM - 5 PM CST
Step 1: Treat
VanaLice™ Lice Killing Gel
See Usage Guidelines to see how much product you will need.
NOTE:
- Use towels to protect child's eyes from treatment and prevent clothes from getting wet.
- Apply Lice Killing Gel thoroughly to DRY HAIR or other affected area.
- Wetting the hair dilutes the treatment making it less effective.
- Also, lice can hold their breath when immersed in water making it difficult for the active ingredient to penetrate the lice.
- For head lice, thoroughly massage Gel into scalp, behind the ears and to the back of the neck. Lice can crawl up and down the shaft of the hair very quickly so it is important to apply the treatment from the roots to the ends of the hair
- Allow product to remain on the hair (or other affected area) for 10 minutes, but no longer.
- Wash area thoroughly with warm water and soap or shampoo
- For head lice, towel dry hair and comb out tangles
- proceed to step 2
- A second treatment must be done in 7 to 10 days to kill any newly-hatched lice.
Step 2: Remove
Lice/Nit Comb
Combing must be done AFTER use of VanaLice™ Lice Killing Gel (Step 1). Combing out eggs & nits* is an
essential step to complete lice elimination.
NOTE:
- A fine-toothed comb or the special lice/nit removing comb (included) must be used to remove lice, eggs, and nits from hair.
- Before starting have on hand
- extra towels
- bobby pins or hair clips
- facial tissues or paper towels
- regular comb for parting and detangling hair
- detangler/conditioner to make combing easier
- Find a distraction for your child (e.g. books, TV, game).
- When combing, use the fine-tooth lice/nit comb provided and make sure the teeth are facing you
- Part hair into sections. Working one section at a time, lift a 1- to 2- inch wide strand of hair
- Place comb as a close to the scalp as possible and comb with a firm, even motion away from scalp, from the roots to the end of the hair (newly-laid eggs are found near the roots of the hair so it is important to get the teeth very close to the scalp). Make sure the teeth are deeply embedded into the strands as you comb through.
- Always comb away from the head.
- Wipe eggs and nits from the teeth of the comb with a tissue or paper towel.
- Remove any remaining nits by hand (using a throw-away-glove).
- Pin back the 1- to 2-inch wide strand you just finished combing with a bobby pin or hair clip.
- Lift up another 1- to 2-inch wide strand and repeat the combing procedure.
- When one section has been combed and pinned, repeat the process for another section of hair. Continue to comb hair 1- to 2-inches at a time until entire head has been combed and pinned.
- Keep the hair damp during combing and re-apply detangler/conditioner as needed.
- After you have combed and pinned the entire head, remove all bobby pins or hair clips and rinse hair thoroughly with warm water. If desired, you may also wash with regular shampoo.
- Discard all the used tissues or paper towels into a bag, seal and dispose to prevent lice from coming back.
*Nits are empty eggshells left behind when lice hatch from eggs.
Step 3 – Control
Head Lice
- Lay small white eggs (nits) on hair shaft close to scalp.
- Nits are most easily found on back of neck or behind ears.
- Disinfect hats, hair ribbons, scarves, coats, towels, and bed linens by machine washing in hot water [above 54°C (130°F)], then using hottest dryer cycle for at least 20 minutes.
- Items that cannot be washed (bedspreads, blankets, pillows, stuffed toys, etc.) should be dry- cleaned or sealed in a plastic bag for 4 weeks, then removed outdoors and shaken out very hard before using again.
- Items that cannot be washed, dry-cleaned, or stored may be sprayed with a product designed for this purpose.
- Soak all combs and brushes in hot water [above 54°C (130°F)] for at least 10 minutes.
- Vacuum all carpets, mattresses, upholstered furniture, and car seats that may have been used by affected people.
Pubic (Crab) Lice
- May be transmitted by sexual contact. Sexual partners should be treated simultaneously to avoid reinfestation.
- Lice are very small and look like brown or grey dots on skin.
- Usually cause intense itching and lay small white eggs (nits) on the hair shaft generally close to the skin surface.
- May be present on the short hairs of groin, thighs, trunk, and underarms, and occasionally on the beard and mustache.
- Disinfect underwear by machine washing in hot water [above 54°C (130°F)], then using hottest dryer cycle for at least 20 minutes.
Body Lice
- Body Lice and their eggs (nits) are generally found in the seams of clothing particularly in waistline and armpit area.
- Body Lice feed on skin then return to clothing to lay their eggs.
- Disinfect clothing by machine washing in hot water [above 54°C (130°F)], then using hottest dryer cycle for at least 20 minutes.
- Do not seal clothing in a plastic bag because nits can remain dormant for up to 30 days.
Daily Checking
TO PREVENT REINFESTATION:
- Carefully inspect infested family member
daily for at least 2 weeks. If you see eggs or nits, repeat Step 2.
- Check other family members for Head Lice. If anyone in the family is infested, follow Step 1 and Step 2. Repeat Step 3 with any articles or items with which they have come into contact.
Checklist
- Home and auto upholstery
- Bike/sport helmets
- Mattresses, pillows, pillowcases
- Coats, scarves, earmuffs, gloves
- Bed linen
- Play mats
- Towels
- Clothing
- Stuffed animals, toys
- Barrettes/hair ornaments
- Headphones
- Hair rollers
- Hats
- Combs and brushes
- Headbands
- Baseball caps