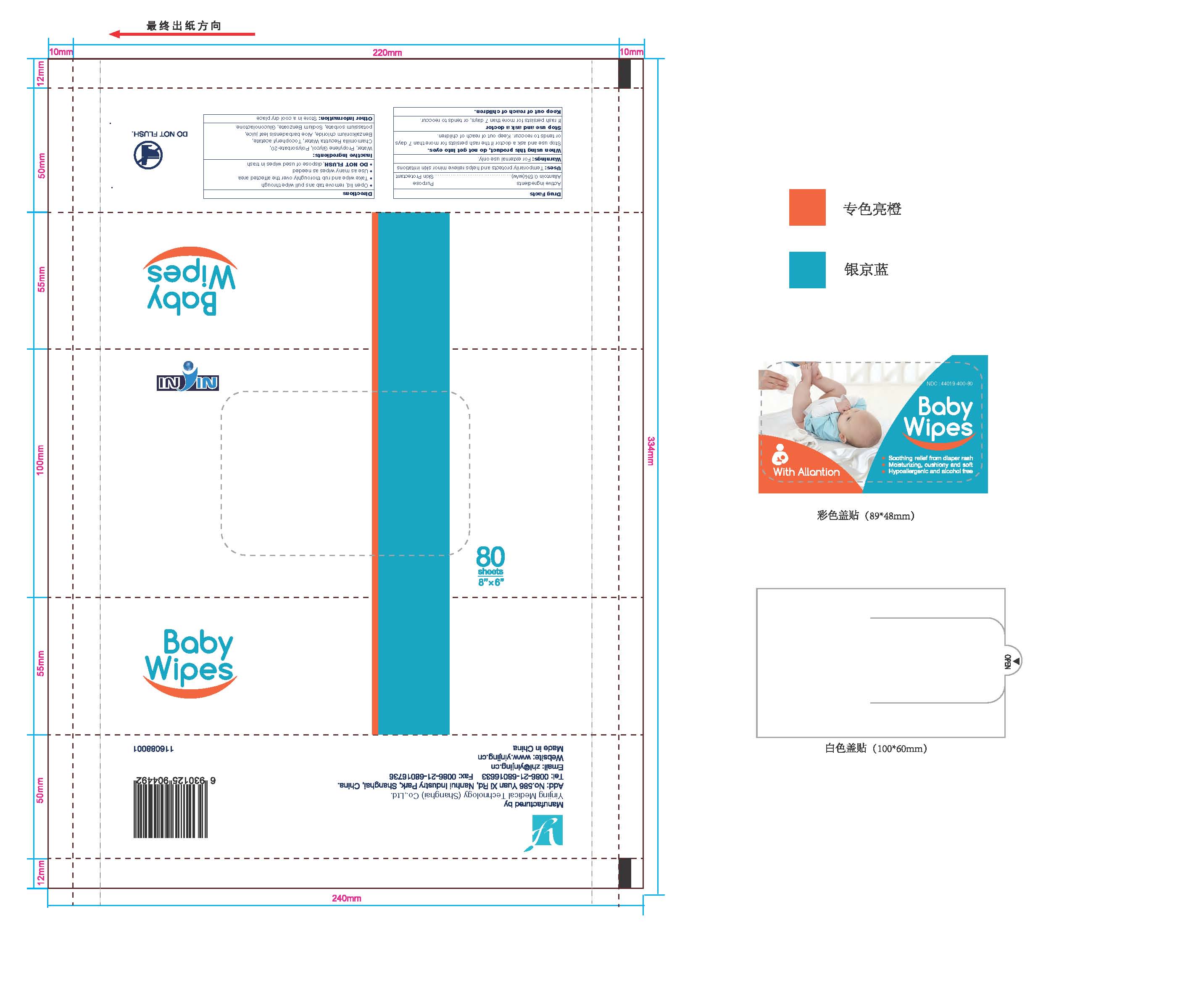
BABY WIPES WITH ALLANTOIN- allantoin liquid
Yinjing Medical Technology (Shanghai) Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Baby Wipes with Allantoin
Uses:
Temporarily protects and helps relieve minor skin irritations
Warnings:
For external use only.
When using this product, do not get into eyes.
Stop use and ask a doctor if the rash persists for more than 7 days,
or tends to reoccur. Keep out of reach of children
Stop use and ask a doctor
if rash persists for more than 7 days, or tends to reoccur.
Keep out of reach of children.
Directions
- Open lid, remove tab ans pull wipe through
- Take wipe and rub thoroughly over the affected area
- Use as many wipes as needed
- DO NOT FLUSH,dispose of used wipes in trash
Inactive Ingredients:
Water, Propylene Glycol, Polysorbate-20, Chamomilla Recutita Water, Tocopheryl acetate, Benzalkonium chloride, Aloe barbadensis leaf juice, potassium sorbate, Sodium Benzoate, Gluconolactone.
Other Information:
Store in a cool dry place
Baby Wipes with Allantion 80 Count (44019-400-80)
Yinjing Medical Technology (Shanghai) Co., Ltd.
