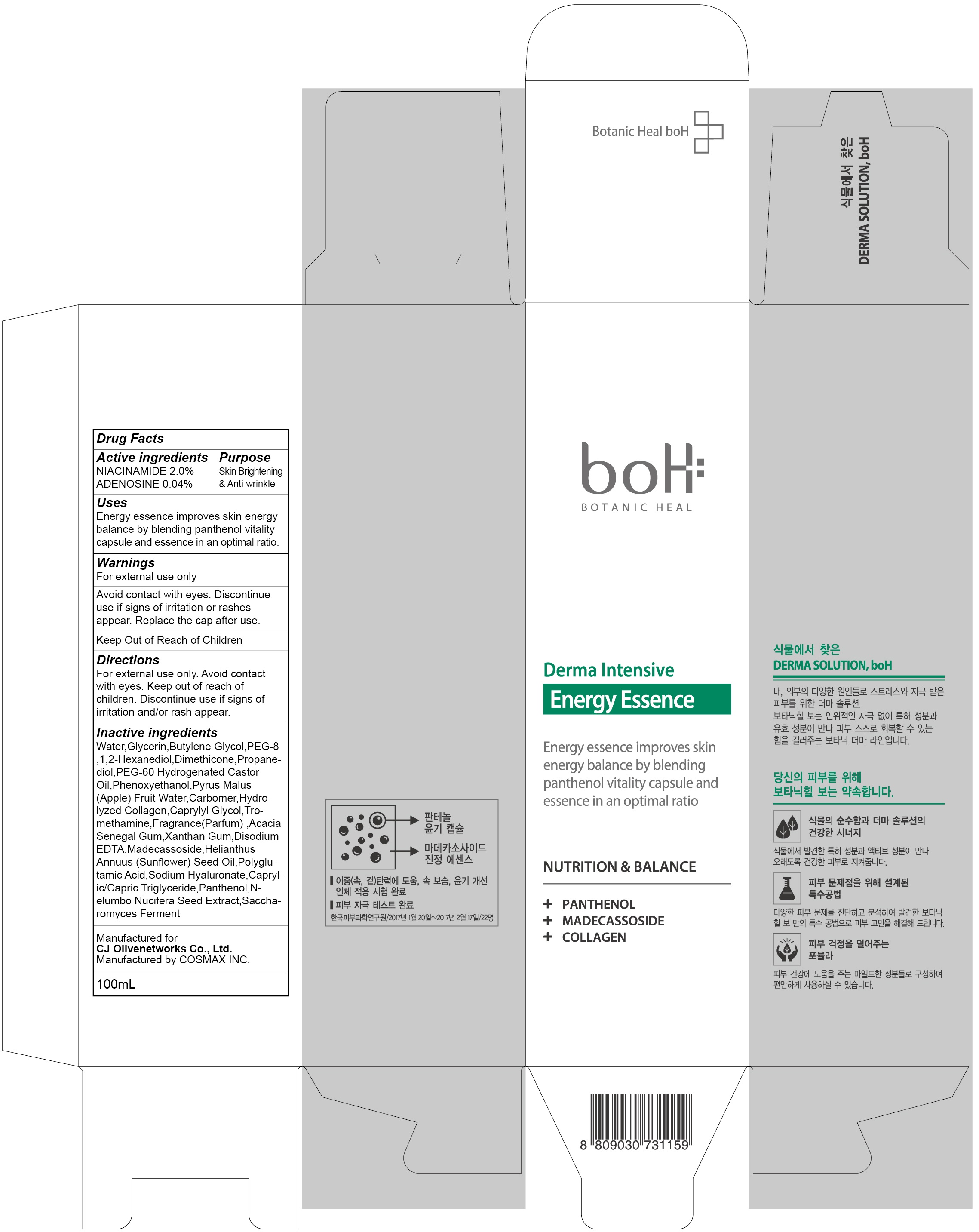
INACTIVE INGREDIENT
Inactive ingredients: Water,Glycerin,Butylene Glycol,PEG-8 ,1,2-Hexanediol,Dimethicone,Propanediol,PEG-60 Hydrogenated Castor Oil,Phenoxyethanol,Pyrus Malus (Apple) Fruit Water,Carbomer,Hydrolyzed Collagen,Caprylyl Glycol,Tromethamine,Fragrance(Parfum) ,Acacia Senegal Gum,Xanthan Gum,Disodium EDTA,Madecassoside,Helianthus Annuus (Sunflower) Seed Oil,Polyglutamic Acid,Sodium Hyaluronate,Caprylic/Capric Triglyceride,Panthenol,Nelumbo Nucifera Seed Extract,Saccharomyces Ferment
WARNINGS
Warnings: For external use only. Avoid contact with eyes. Discontinue use if signs of irritation or rashes appear. Replace the cap after use. Keep Out of Reach of Children.
Uses
Uses: Energy essence improves skin energy balance by blending panthenol vitality capsule and essence in an optimal ratio.