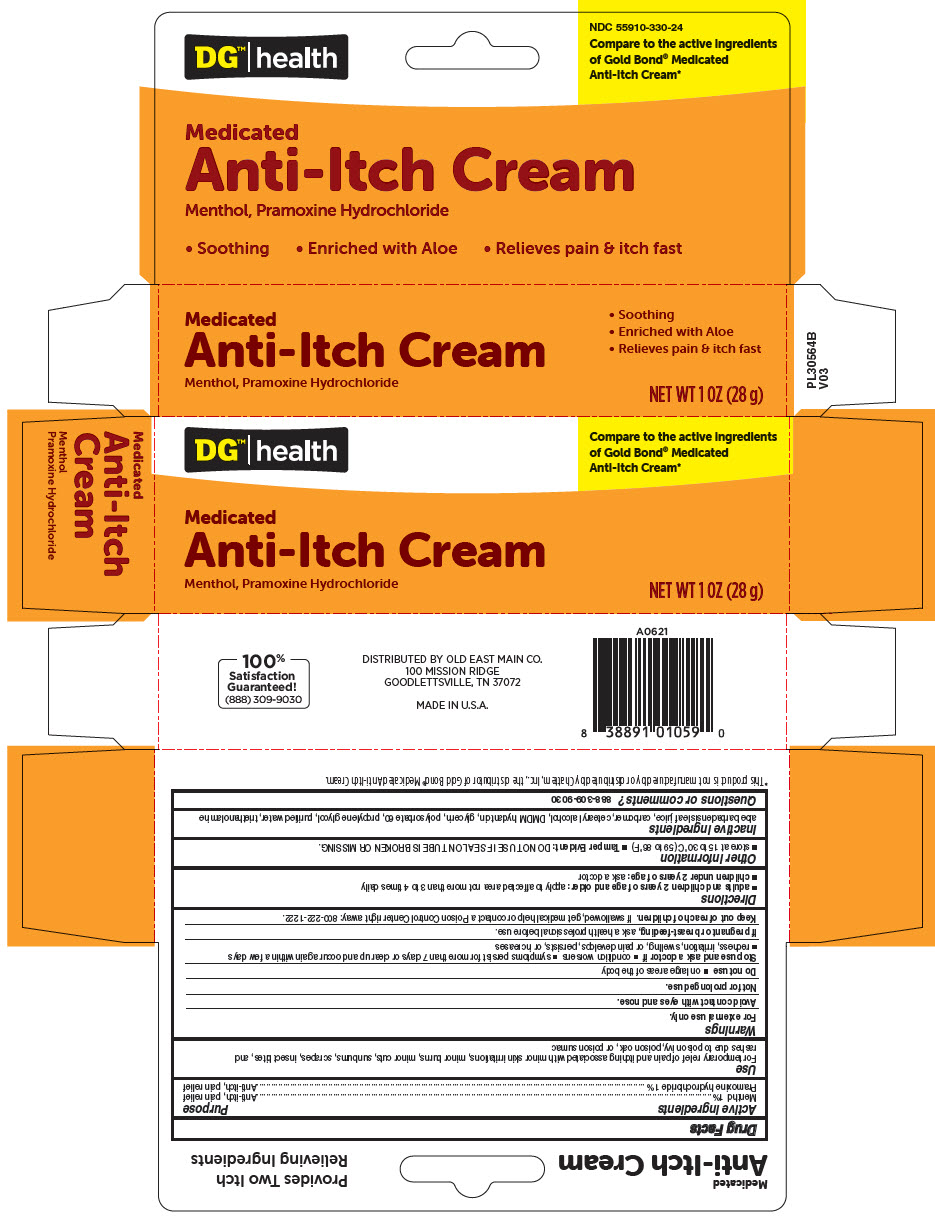
DG HEALTH MEDICATED ANTI-ITCH ANALGESIC- menthol, unspecified form and pramoxine hydrochloride cream
DOLGENCORP, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| Active ingredients | Purpose |
| Menthol 1% | Anti-itch, pain relief |
| Pramoxine hydrochloride 1% | Anti-itch, pain relief |
Use
For temporary relief of pain and itching associated with minor skin irritations, minor burns, minor cuts, sunburns, scrapes, insect bites, and rashes due to poison ivy, poison oak, or poison sumac
Warnings
For external use only.
Avoid contact with eyes and nose.
Not for prolonged use.
Do not use
- on large areas of the body
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
- redness, irritation, swelling, or pain develops, persists, or increases
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away: 800-222-1222.
Directions
-
adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
-
children under 2 years of age: ask a doctor
Other information
- store at 15 to 30°C (59 to 86°F)
-
Tamper Evident: DO NOT USE IF SEAL ON TUBE IS BROKEN OR MISSING.
Inactive ingredients
aloe barbadensis leaf juice, carbomer, cetearyl alcohol, DMDM hydantoin, glycerin, polysorbate 60, propylene glycol, purified water, triethanolamine
Questions or comments?
888-309-9030
DISTRIBUTED BY OLD EAST MAIN CO.
100 MISSION RIDGE
GOODLETTSVILLE, TN 37072
PRINCIPAL DISPLAY PANEL - 28 g Tube Carton
DG™| health
Compare to the active ingredients
of Gold Bond® Medicated
Anti-Itch Cream*
Medicated
Anti-Itch Cream
Menthol, Pramoxine Hydrochloride
NET WT 1 OZ (28 g)