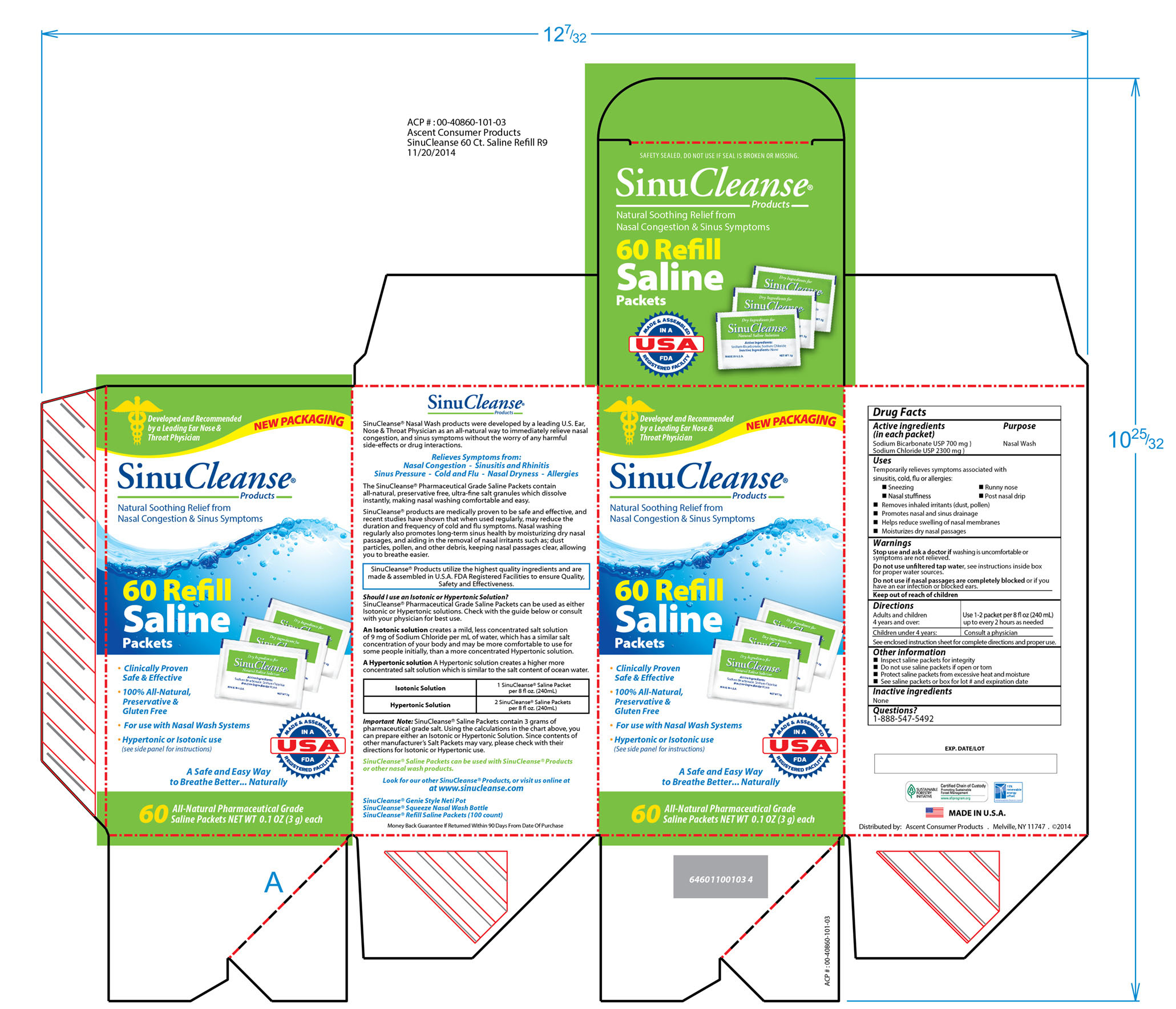
Uses
Temporarily relieves symptoms associated with
sinusitis, cold, flu or allergies:
-Sneezing
-Runny nose
-Nasal stuffiness
-Post nasal drip
-Removes inhaled irritants (dust, pollen)
-Promotes nasal and sinus drainage
-Helps reduce swelling of nasal membranes
-Moisturizes dry nasal passages
Warnings
Stop use and ask a doctor if washing is uncomfortable or symptoms
are not relieved.
Do not use unfiltered tap water, see instructions inside box
for proper water sources.
Do not use if nasal passages are completely blocked or if you have
an ear infection or blocked ears.
When using this product:
-Use by only one person
-Wash with soap and water after each use
-Top rack of dishwasher safe
-Do not heat in microwave
Directions
Adults and children 4 years and over: use 1/2 - 1 packet per 4 fl oz (120 mL)up to every two hours as needed
Children under 4 years: Consult a physician
See enclosed instruction sheet for complete directions and proper use