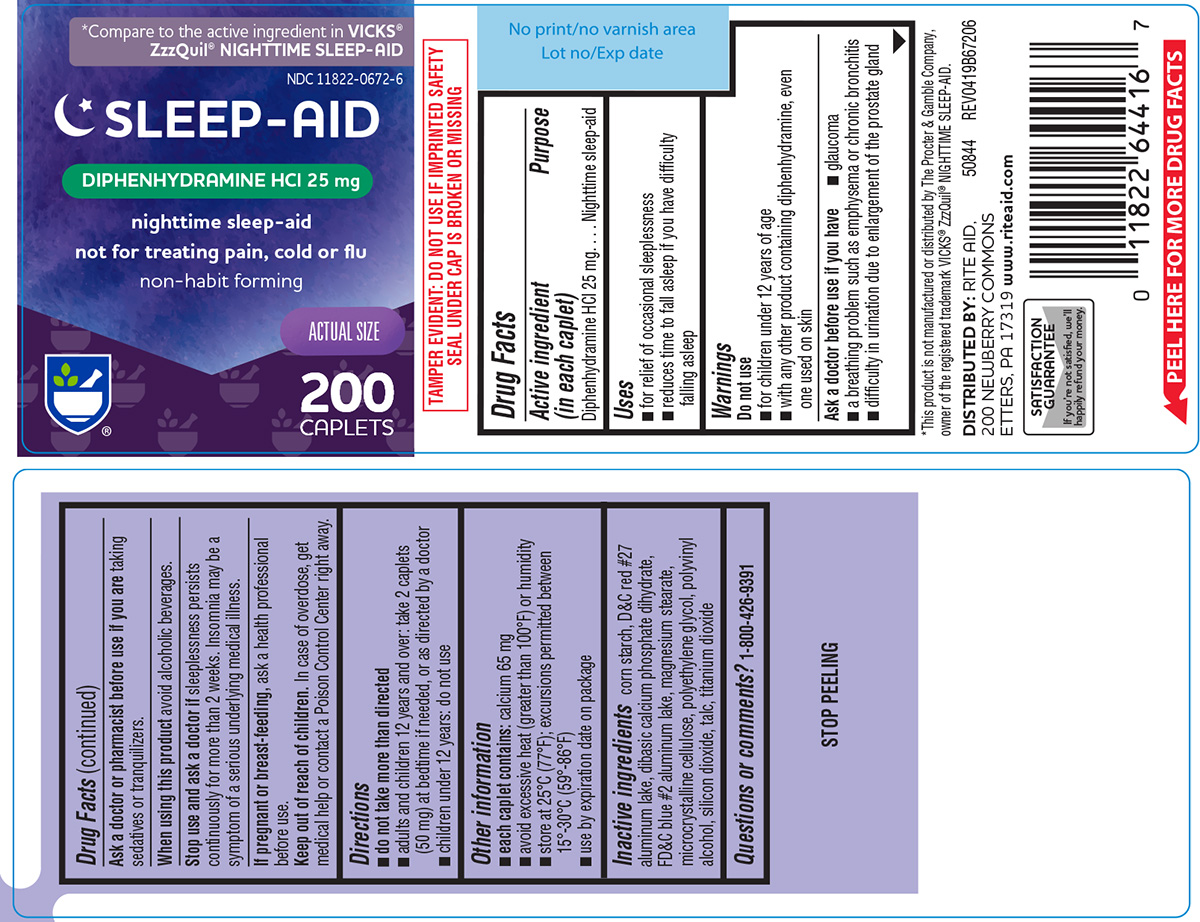
Uses
- for relief of occasional sleeplessness
- reduces time to fall asleep if you have difficulty falling asleep
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on skin
- for children under 12 years of age
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Directions
- do not take more than directed
- adults and children 12 years and over: take 2 caplets (50 mg) at bedtime if needed, or as directed by a doctor
- children under 12 years: do not use
Other information
- each caplet contains: calcium 65 mg
- avoid excessive heat (greater than 100ºF) or humidity
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- use by expiration date on package
Inactive ingredients
corn starch, D&C red #27 aluminum lake, dibasic calcium phosphate dihydrate, FD&C blue #2 aluminum lake, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, silicon dioxide, talc, titanium dioxide
Principal display panel
*Compare to the active ingredient in VICKS®
ZzzQuil® NIGHTTIME SLEEP-AID
NDC 11822-0672-6
SLEEP-AID
DIPHENHYDRAMINE HCl 25 mg
nighttime sleep-aid
not for treating pain, cold or flu
non-habit forming
200
CAPLETS
ACTUAL SIZE
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY
SEAL UNDER CAP IS BROKEN OR MISSING
DISTRIBUTED BY: RITE AID, 200 NEWBERRY COMMONS,
ETTERS, PA 17319
www.riteaid.com
*This product is not manufactured or distributed by The Procter & Gamble Company,
owner of the registered trademark VICKS® ZzzQuil® NIGHTTIME SLEEP-AID.
50844 REV0419B67206
SATISFACTION
GUARANTEE
if you're not satisfied, we'll
happily refund your money.
Rite Aid 44-672