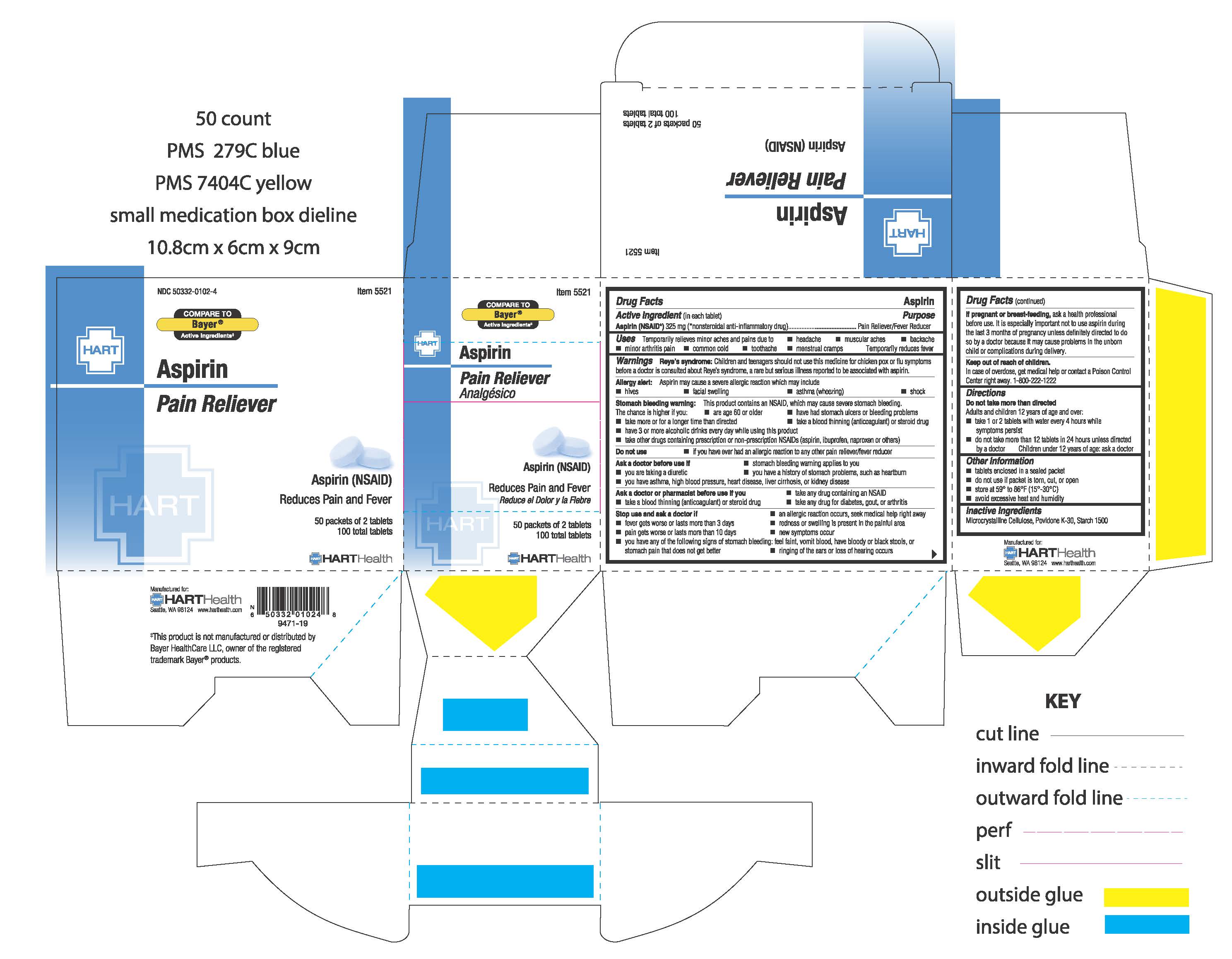
Uses:
Temporarily relieves minor aches and pains due to:
- headache
- backache
- muscular aches
- minor arthritis pain
- common cold
- toothache
- menstrual cramps
Temporarily reduces fever
Warnings:
Reye's Syndrome: Children and teenagers should not use this medicine for chicken pox or flu symptoms before a doctor is consulted about Reye's syndrome, a rare but serious illness reported to be associated with aspirin
Allergy altert: Aspirin may cause a several allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulers or bleeding problems
- take more or for a longer time than directed
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or non-prescription NSAIDs (aspirin, ibuprofen, naproxen, others)
- have 3 or more alcholic drinks every day while using this product
Ask a doctor before use if:
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you are taking a diuretic
- you have asthma, high blood pressure, heart disease, liver cirrhosis, or kidney disease
Ask a doctor or pharmacist before use if you:
- take any other drug containing an NSAID
- take a blood thinning (anticoagulant) or steroid drug
- take any drug for diabetes, gout, or arthritis
Stop use and ask a doctor if:
- an allergic reaction occurs, seek medical help right away
- fever gets worse or lasts more than 3 days
- pain gets worse or lasts more than 10 days
- redness or swelling is present in the painful area
- ringing in the ears or loss of hearing occurs
- new symptoms occur
- you have any of the following signs of stomach bleeding: feel faint, vomit blood, have bloody or black stools, or stomach pain that does not get better
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. 1-800-222-1222