Active ingredients**
Arnicare Gel: Arnica Montana 1X HPUS 7%
Arnica 30C pellets: (in each pellet) Arnica Montana 30C HPUS (0.443 mg)
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
Uses*
temporarily relieves muscle pain and stiffness due to:
- minor injuries
- overexertion
- falls
reduces symptoms of bruising such as:
- pain
- swelling
- discoloration
Purpose*
Arnicare Gel: Arnica Montana 1X HPUS 7% ..... Relieves muscle pain & stiffness, swelling from injuries, discoloration from bruises
Arnica 30C Pellets: Arnica montana 30C HPUS .......... Relieves muscle pain & stiffness, swelling from injuries, discoloration from bruises
When using this product
- avoid contact with eyes, mucous membranes, wounds, damaged or irritated skin
- use only as directed
- dryness or irritation may occur
- do not tightly wrap or bandage the treated area
- do not apply heat or ice to the treated area immediately before or after use.
Stop use and ask a doctor if
condition persists for more than 3 days or worsens
symptoms clear up and occur again within a few days.
ARNICA 30C PELLETS:
Stop use and ask a doctor if symptoms persist for more than 3 days or worsen.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
ARNICARE GEL: Apply a thin layer of Arnicare Gel to affected area as soon as possible after minor injury. Repeat 3 times a day or as needed. If heat or ice is applied, wait 5 minutes before applying Arnicare Gel.
ARNICA 30C PELLETS: At the onset of symptoms, dissolve 5 pellets under the tongue 3 times a day until symptoms are relieved or as directed by a doctor.
ARNICARE GEL: alcohol, carbomer, purified water, sodium hydroxide
ARNICA 30C PELLETS: lactose, sucrose.
do not use if glued carton end flaps are open, if the tube seal is broken, or if pellet dispenser seal is broken
Quickly absorbed, non-greasy, fragrance-free, non-drowsy, no known drug interactions
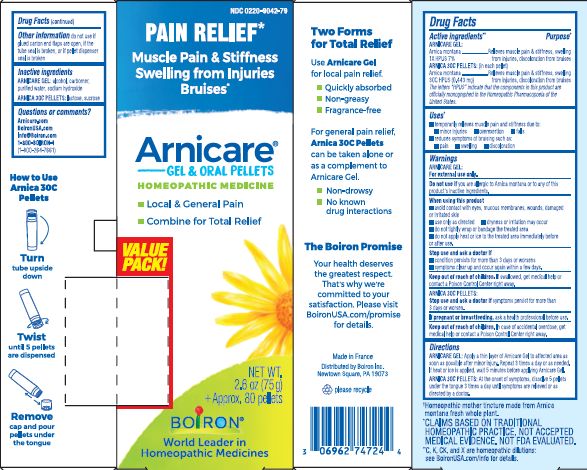
Pain Relief*
Muscle Pain & Stiffness Swelling from Injuries Bruises*
Local & General Pain
Combine for Total Relief
Gel: 1 tube 2.6 oz (75g)
Pellets: + approx. 80 pellets
How to dispense pellets? Turn tube upside down. Twist until 5 pellets are dispensed into cap. Carefully remove the cap and use it to pour pellets under the tongue.
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.