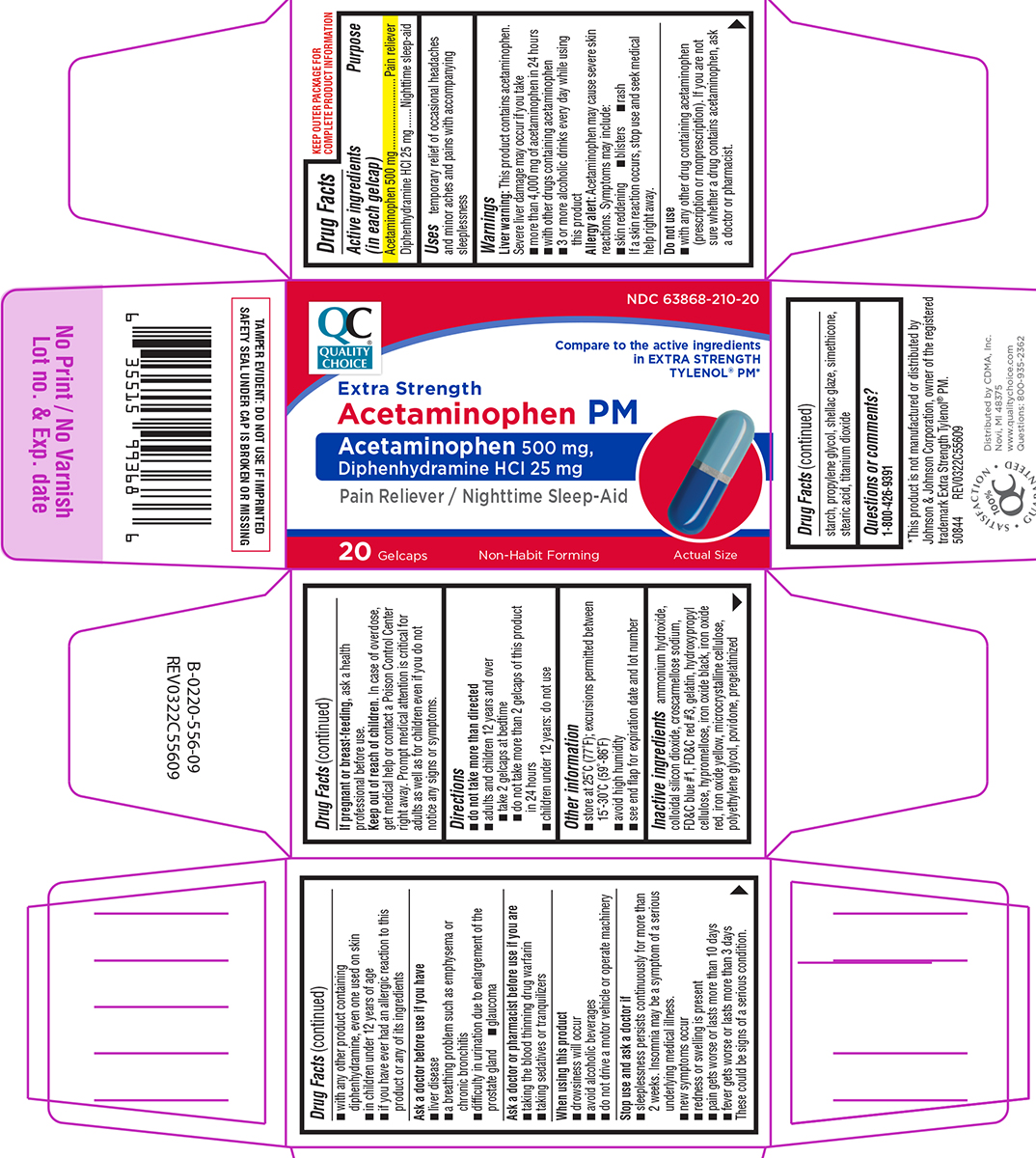
Uses
temporary relief of occasional headaches and minor aches and pains with accompanying sleeplessness
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with any other product containing diphenhydramine, even one used on skin
- in children under 12 years of age
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver disease
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- drowsiness will occur
- avoid alcoholic beverages
- do not drive a motor vehicle or operate machinery
Stop use and ask a doctor if
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
- new symptoms occur
- redness or swelling is present
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
These could be signs of a serious condition.
Directions
- do not take more than directed
- adults and children 12 years and over
- take 2 gelcaps at bedtime
- do not take more than 2 gelcaps of this product in 24 hours
- children under 12 years: do not use
Other information
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- avoid high humidity
- see end flap for expiration date and lot number
Inactive ingredients
ammonium hydroxide, colloidal silicon dioxide, croscarmellose sodium, FD&C blue #1, FD&C red #3, gelatin, hydroxypropyl cellulose, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, microcrystalline cellulose, polyethylene glycol, povidone, pregelatinized starch, propylene glycol, shellac glaze, simethicone, stearic acid, titanium dioxide
Principal Display Panel
QC®
QUALITY
CHOICE
NDC 63868-210-20
Compare to the active ingredients
in EXTRA STRENGTH
TYLENOL® PM*
Extra Strength
Acetaminophen PM
Acetaminophen 500 mg,
Diphenhydramine HCl 25 mg
Pain Reliever / Nighttime Sleep-Aid
20 Gelcaps
Non-Habit Forming
Actual Size
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by
Johnson & Johnson Corporation, owner of the registered
trademark Extra Strength Tylenol® PM.
50844 REV0322C55609
Distributed by CDMA, Inc.
Novi, MI 48375
www.qualitychoice.com
Questions: 800-935-2362
Quality Choice 44-556