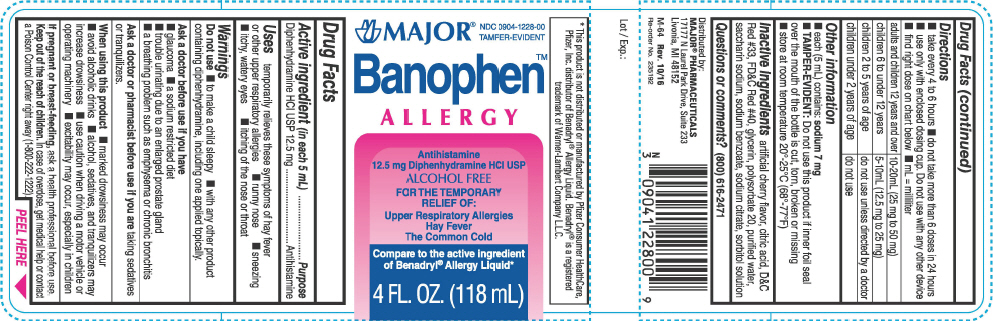
Uses
temporarily relieves these symptoms of hay fever or other upper respiratory allergies
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, including one applied topically.
Ask a doctor before use if you have
- glaucoma
- a sodium restricted diet
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Directions
- take every 4 to 6 hours
- do not take more than 6 doses in 24 hours
- use only with enclosed dosing cup. Do not use with any other device
- find right dose on chart below
- mL = milliliter
| adults and children 12 years and over | 10-20mL (25 mg to 50 mg) |
| children 6 to under 12 years | 5-10mL (12.5 mg to 25 mg) |
| children 2 to 5 years of age | do not use unless directed by a doctor |
| children under 2 years of age | do not use |
Other information
- each (5 mL) contains: sodium 7 mg
- TAMPER-EVIDENT: Do not use this product if inner foil seal over the mouth of the bottle is cut, torn, broken or missing
- store at room temperature 20°-25°C (68°-77°F)
Inactive ingredients
artificial cherry flavor, citric acid, D&C Red #33, FD&C Red #40, glycerin, polysorbate 20, purified water, saccharin sodium, sodium benzoate, sodium citrate, sorbitol solution
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Label
MAJOR
®
NDC 0904-1228-00
TAMPER-EVIDENT
Banophen™
ALLERGY
Antihistamine
12.5 mg Diphenhydramine HCl USP
ALCOHOL FREE
FOR THE TEMPORARY
RELIEF OF:
Upper Respiratory Allergies
Hay Fever
The Common Cold
Compare to the active ingredient
of Benadryl
® Allergy Liquid*
4 FL. OZ. (118 mL)