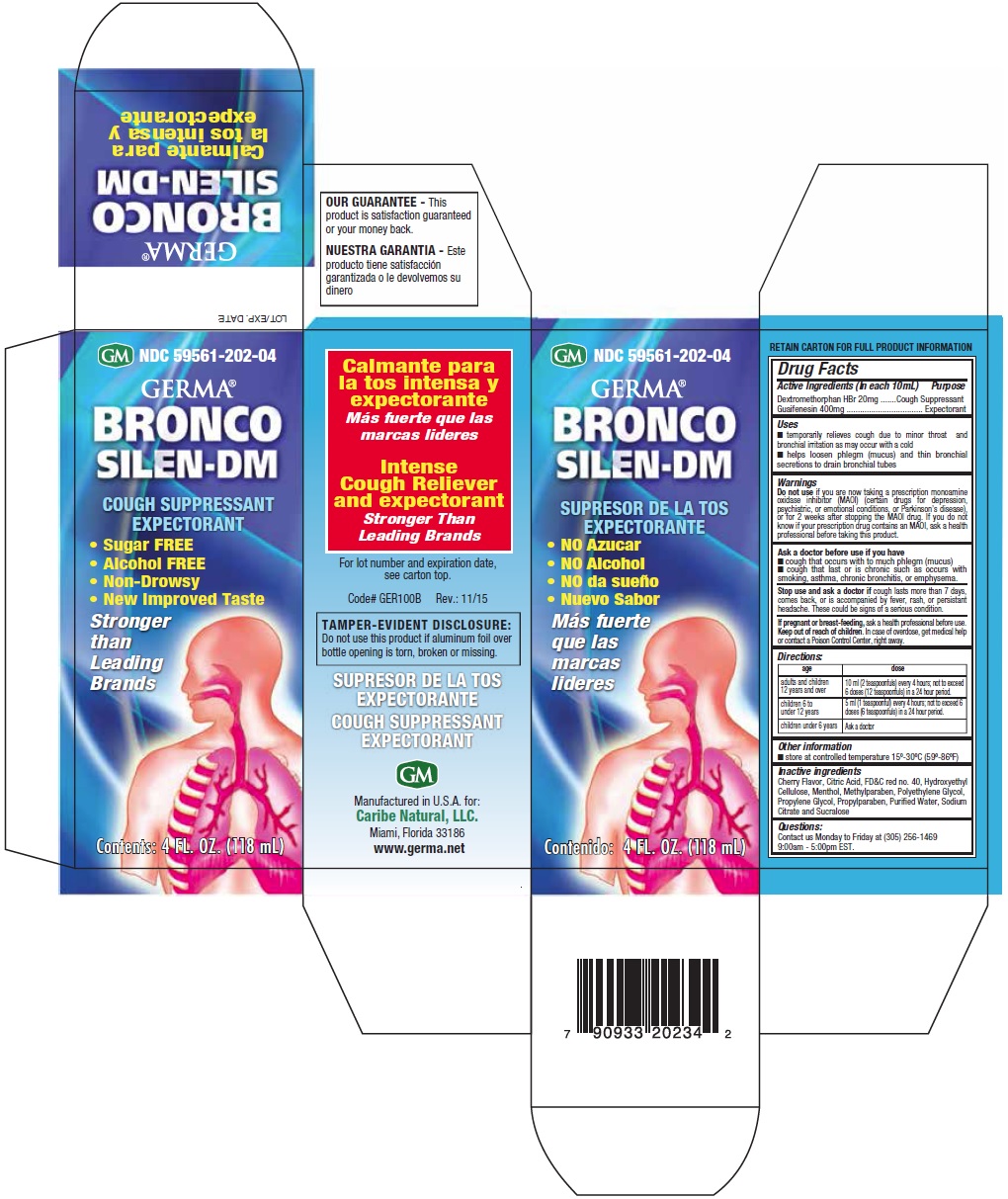
Uses
- temporarily relieves cough due to minor throat and bronchial irritation may occur with a cold
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a health professional before taking this product.
Ask a doctor before use if you have
- cough that occurs with to much phlegm (mucus)
- cough that last or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Stop use and ask a doctor if cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
Keep out of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away.
Directions:
| age | dose |
|---|---|
| adults and children 12 years and over | 10 ml (2 teaspoonfuls) every 4 hours; not to exceed 6 doses (12 teaspoonfuls) in a 24 hour period. |
| children 6 to under 12 years | 5 ml (1 teaspoonful) every 4 hours; not to exceed 6 doses (6 teaspoonfuls) in a 24 hour period. |
| children under 6 years | Ask a doctor |