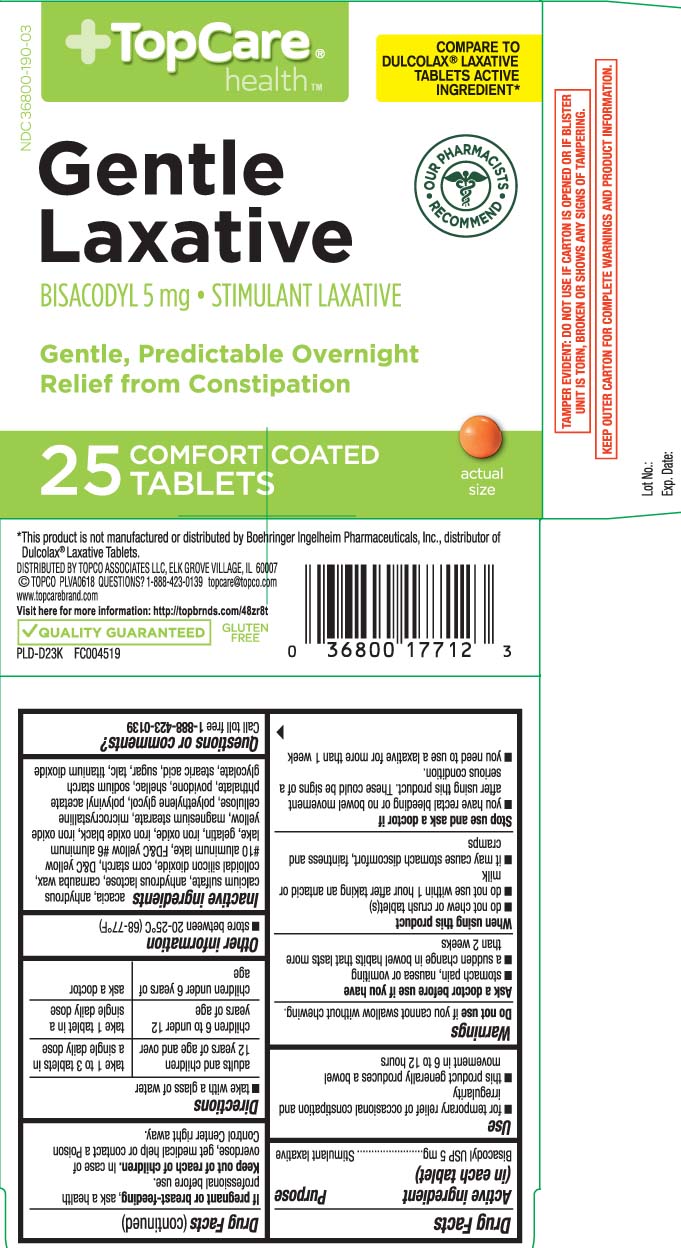
Use
- for temporary relief of occasional constipation and irregularity
- this product generally produces a bowel movement in 6 to 12 hours
Warnings
Ask a doctor before use if you have
- stomach pain, nausea or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
When using this product
- do not chew or crush tablet(s)
- do not use within 1 hour after taking an antacid or milk
- it may cause stomach discomfort, faintness and cramps
Directions
- take with a glass of water
| adults and children 12 yers of age and over | take 1 to 3 tablets in a single daily dose |
| children 6 to under 12 years of age | take 1 tablet in a single daily dose |
| children under 6 years of age | ask a doctor |
Inactive ingredients
acacia, anhydrous calcium sulfate, anhydrous lactose, carnauba wax, colloidal silicon dioxide, corn starch, D&C yellow #10 aluminum lake, FD&C yellow #6 aluminum lake, gelatin, iron oxide, iron oxide black, iron oxide yellow (iron oxide ochre), magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl acetate phthalate, povidone, shellac, sodium starch glycolate, stearic acid, sugar, talc, and titanium dioxide
Principal Display Panel
COMPARE TO DULCOLAX® LAXATIVE TABLETS ACTIVE INGREDIENT*
Gentle Laxative
BISACODYL 5 mg • STIMULANT LAXATIVE
Gentle, Predictable Overnight Relief from Constipation
COMFORT COATED TABLETS
*This product is not manufactured or distributed by Boehringer Ingelheim Pharmaceuticals, Inc.,distributor of Dulcolax® Laxative Tablets.
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
DISTRIBUTED BY TOPCO ASSOCIATES LLC
ELK GROVE VILLAGE, IL 60077
QUESTIONS? 1-888-423-0139