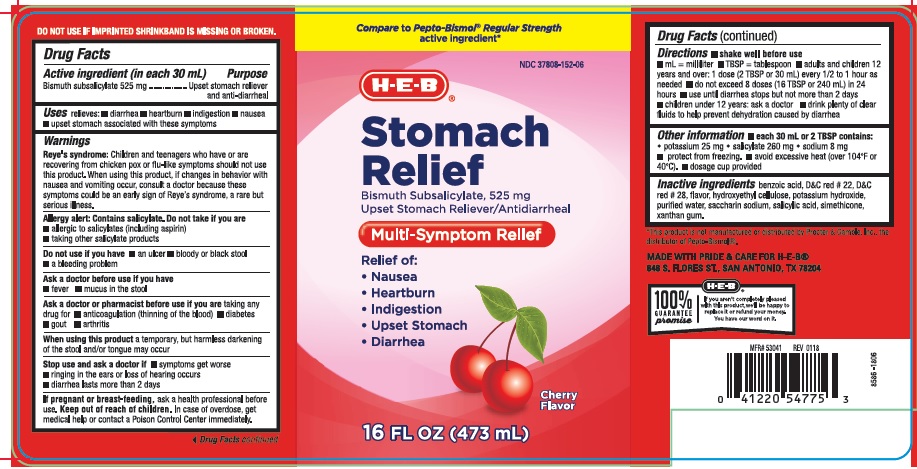
WARNINGS
Reye's Syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's Syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are
- allergic to salicylates (including aspirin)
- taking other salicylate products
ASK A DOCTOR OR PHARMACIST BEFORE USE IF YOU ARE
taking any drug for
- anticoagulation (thinning of the blood)
- diabetes
- gout
- arthritis
STOP USE AND ASK DOCTOR IF
- symptoms get worse
- ringing in the ears or loss of hearing occurs
- diarrhea lasts more than 2 days
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center immediately.
DIRECTIONS
- shake well before use
- mL = milliliter
- TBSP = tablespoon
- adults and children 12 years and over: 1 dose (2 TBSP or 30 mL) every 1/2 to 1 hour as needed
- do not exceed 8 doses (16 TBSP or 240 mL) in 24 hours
- use until diarrhea stops but not more than 2 days
- children under 12 years: ask a doctor
- drink plenty of fluids to help prevent dehydration caused by diarrhea
OTHER INFORMATION
- each 30 mL or 2 TBSP contains:
- potassium 25 mg
- salicylate 260 mg
- sodium 8 mg
- protect from freezing
- avoid excessive heat (over 104oF or 40oC)
- dosage cup provided
INACTIVE INGREDIENTS
benzoic acid, D&C red # 22, D&C red # 28, flavor, hydroxyethyl cellulose, potassium hydroxide, purified water, saccharin sodium, salicylic acid, simethicone, xanthan gum
PRINCIPAL DISPLAY PANEL
Compare to Pepto-Bismol® Regular strength active ingredient*
NDC 37808-152-06
HEB
Stomach Relief
Bismuth Subsalicylate 525 mg
Upset stomach reliever/Anti-diarrheal
Multi-Symptom Relief
Relief of:
- Nausea
- Heartburn
- Indigestion
- Upset stomach
- Diarrhea
Cherry Flavor
16 FL OZ (473 mL)