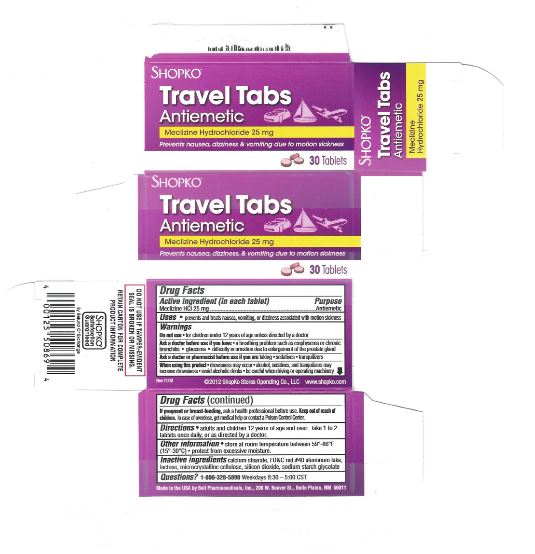
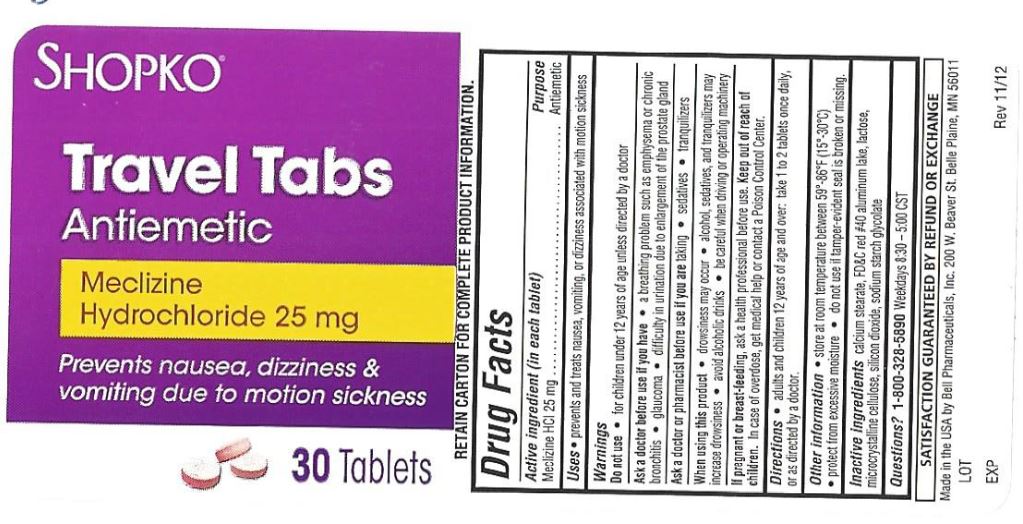
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urinating due to an enlargement of the prostate gland
Directions
-
take first dose one hour before starting activity
-
adults and children 12 years and over: 1 to 2 tablets once daily, or as directed by a doctor
Inactive ingredients
calcium stearate, FD&C red #40 aluminum lake, lactose, microcrystalline cellulose, silicon dioxide, Sodium starch glycolate
Other Information
- store at room temperature between 59º - 86ºF (15 - 30ºC)
- protect from excessive moisture.
PRINCIPAL DISPLAY PANEL
Compare to Dramamine® LESS DROWSY FORMULA active ingredients*
DISCOUNT drug mart FOOD FAIR
less drowsy
motion sickness relief tabs
Meclizine Hydrochloride
- antiemetic
provides Up To 24 Hours of Motion Sickness Protection
prevents nausea, dizziness, vomiting
8 TABLETS (25 mg EACH) new smaller size
Retain carton for complete product information.
Distributed by: Drug Mart-Food Fair
211 Commerce Dr., Medina, Ohio 44256
Questions?: 866-467-2748