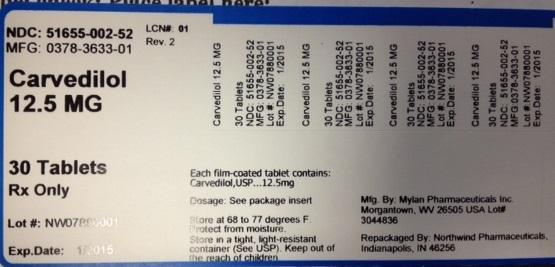
NDC: 51655-002-52
MFG: 0378-3633-01
Carvedilol 12.5 MG
30 Tablets
RX only
Lot#: NW07880001
Exp. Date: 1/2015
Each film-coated tablet contains: Carvedilol, USP...12.5mg
Dosage: See package insert
Store at 68 to 77 degrees F.
Protect from moisture.
Store in a tight, light-resistant container (See USP).
Keep out of the reach of children.
Mfg. by: Mylan Pharmaceuticals Inc. Morgantown, WV 26505 USA Lot# 3044836
Repackaged By: Northwind Pharmaceuticals, Indianapolis, IN 46256