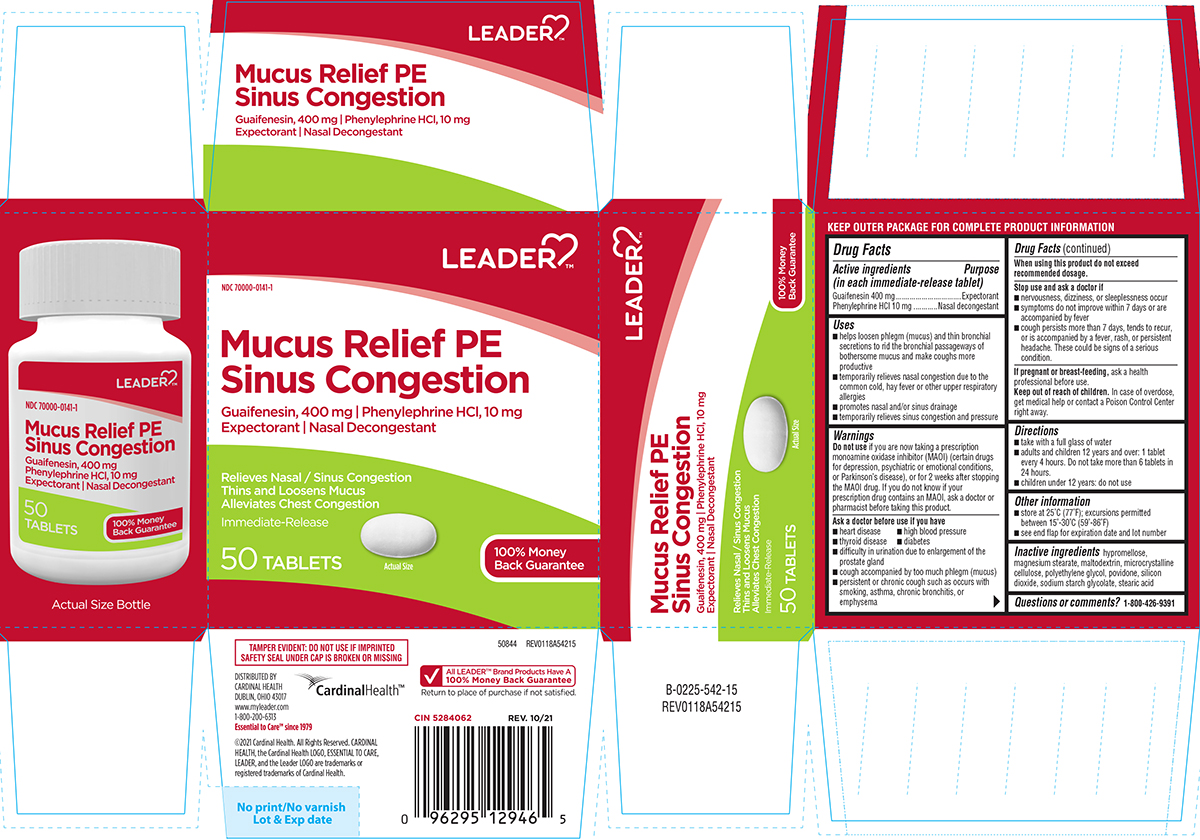
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- promotes nasal and/or sinus drainage
- temporarily relieves sinus congestion and pressure
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- difficulty in urination due to enlargement of the prostate gland
- cough accompanied by too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Directions
- take with a full glass of water
- adults and children 12 years and over: 1 tablet every 4 hours. Do not take more than 6 tablets in 24 hours.
- children under 12 years: do not use
Other information
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, povidone, silicon dioxide, sodium starch glycolate, stearic acid
Principal Display Panel
NDC 70000-0141-1
LEADER™
Mucus Relief PE
Sinus Congestion
Guaifenesin, 400 mg | Phenylephrine HCl, 10 mg
Expectorant | Nasal Decongestant
Relieves Nasal / Sinus Congestion
Thins and Loosens Mucus
Alleviates Chest Congestion
Immediate-Release
50 TABLETS
Actual Size
100% Money Back Guarantee
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
CardinalHealth™
DISTRIBUTED BY
CARDINAL HEALTH
DUBLIN, OHIO 43017
www.myleader.com
1-800-200-6313
Essential to Care™ since 1979
© 2021 Cardinal Health. All Rights Reserved. CARDINAL
HEALTH, the Cardinal Health LOGO, ESSENTIAL TO CARE,
LEADER, and the Leader LOGO are trademarks or
registered trademarks of Cardinal Health.
ALL LEADER™ Brand Products Have A
100% Money Back Guarantee
Return to place of purchase if not satisfied.
50844 REV0118A54215
Leader 44-542