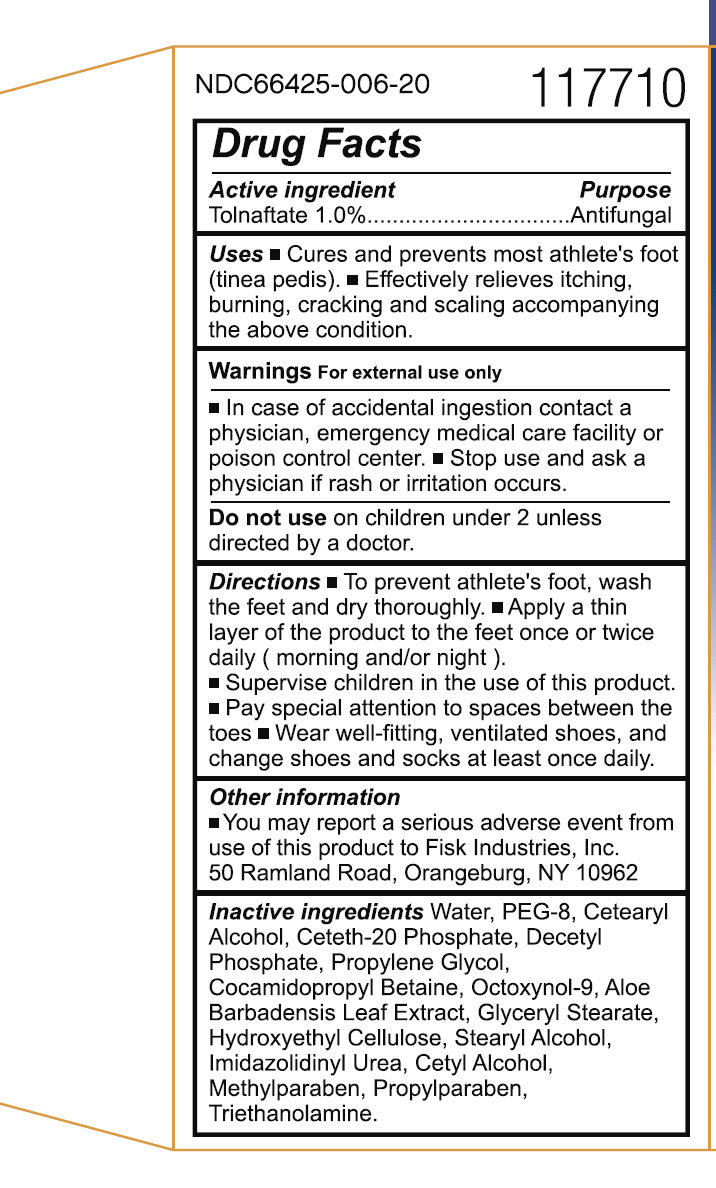
Active ingredient
Active ingredient Purpose
Tolnaftate 1.0%.................................Antifungal
Uses
- Cures and prevents most athlete's foot (tinea pedis). - Effectively relieves itching, burning, cracking and scaling accompanying the above condition.
Keep out of reach of children
- In case of accidental ingestion contact a physician, emergency medical care facility or poison control center.
Directions
- To prevent athlete's foot, wash the feet and dry thoroughly. - Apply a thin layer of the product to the feet once or twice daily ( morning and/or night ). - Supervise children in the use of this product. - Pay special attention to spaces between the toes - Wear well-fitting, ventilated shoes, and change shoes and socks at least once daily.
Other information
- You may report a serious adverse event from use of this product to Fisk Industries, Inc. 50 Ramland Road, Orangeburg, NY 10962
Inactive ingredients
Water, PEG-8, Cetearyl Alcohol, Ceteth-20 Phosphate, Decetyl Phosphate, Propylene Glycol, Cocamidopropyl Betaine, Octoxynol-9, Aloe Barbadensis Leaf Extract, Glyceryl Stearate, Hydroxyethyl Cellulose, Stearyl Alcohol, Imidazolidinyl Urea, Cetyl Alcohol, Methylparaben, Propylparaben, Triethanolamine.
Description
NDC66425-006-20 117710
BIORIUS sprl, 2 rue René Descartes B-7000 Mons, Belgium DISTRIBUTED BY FISK INDUSTRIES ORANGEBURG, NY 10962, USA Made in U.S.A. LOT # 0326BR 7 20817 30326 1 PART # 0326FC
FUNGUS RX PUISSANCE MAXIMUM SOLUTION ANTIFONGIQUE TUE LES GERMES QUI CAUSENT LES INFECTIONS FONGIQUES PRO 30 ml / Fl. Oz. (U.S.) e
BARIELLE PROFESSIONAL MAXIMUM STRENGTH FUNGUS RX ANTIFUNGAL SOLUTION KILLS GERMS THAT CAN CAUSE FUNGAL INFECTIONS PRO 30 ml / Fl. Oz. (U.S.) e BIORIUS sprl, 2 rue René Descartes B-7000 Mons, Belgium DISTRIBUTED BY FISK INDUSTRIES ORANGEBURG, NY 10962, USA Made in U.S.A. LOT #