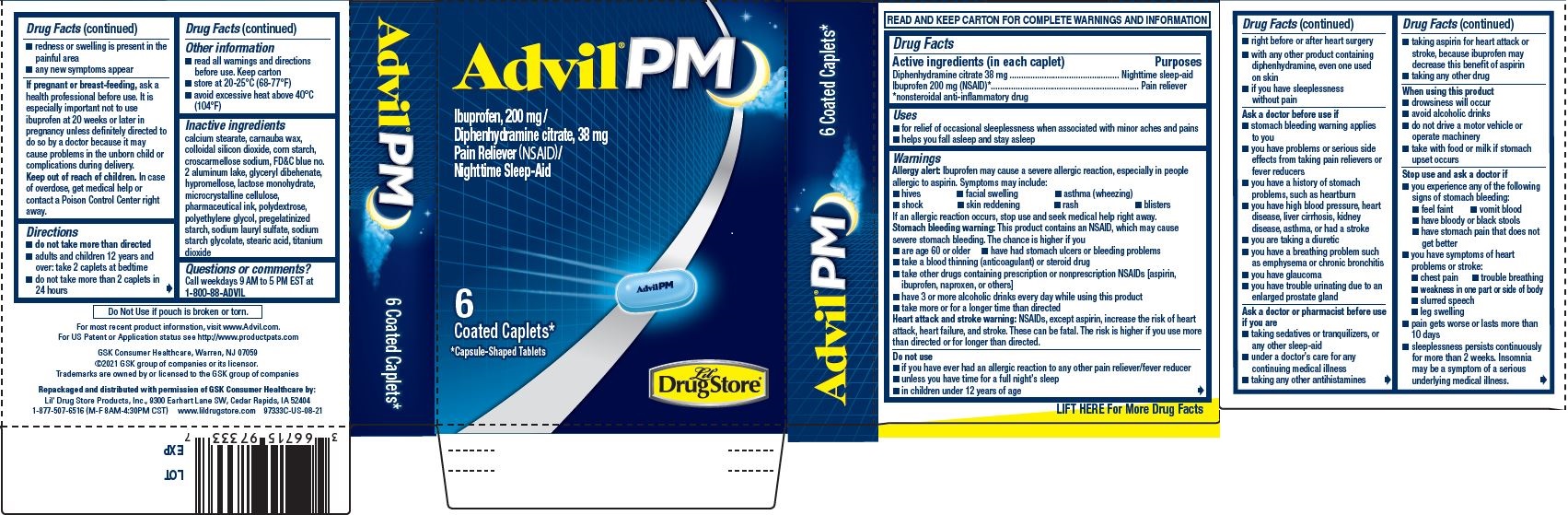
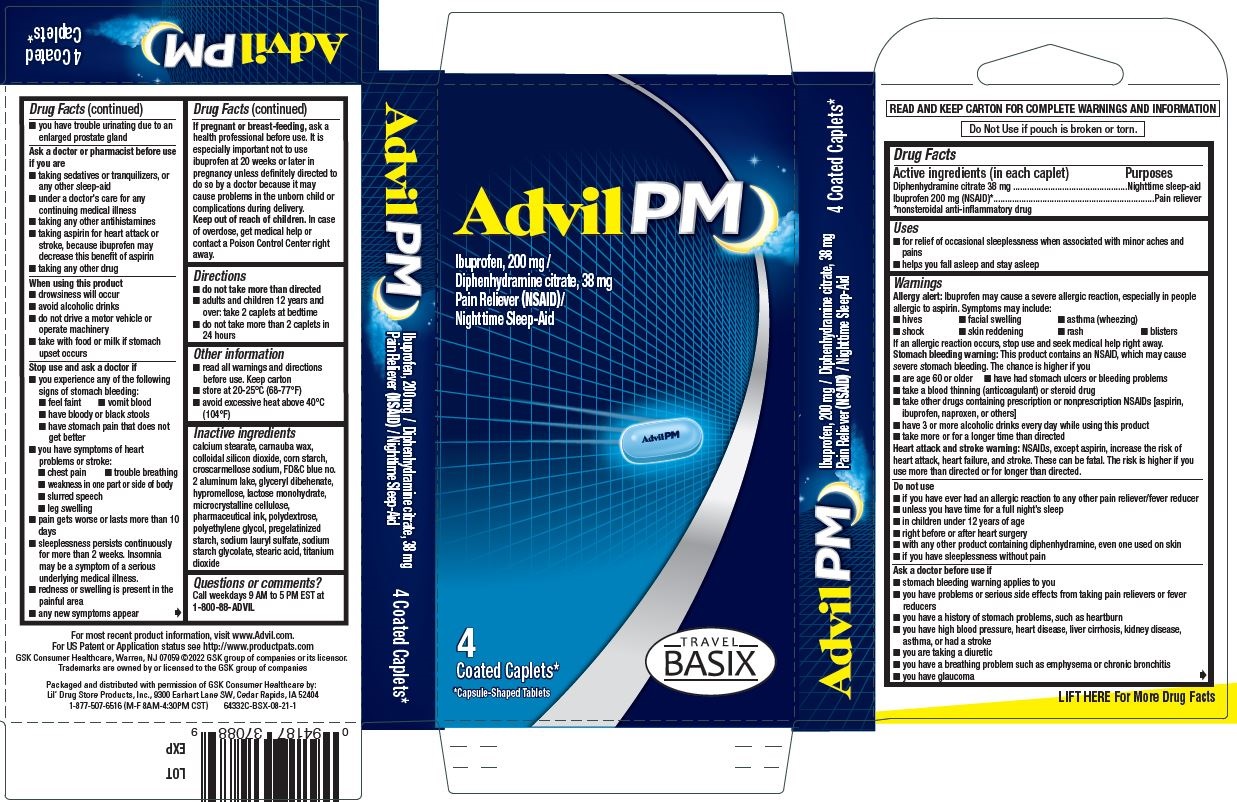
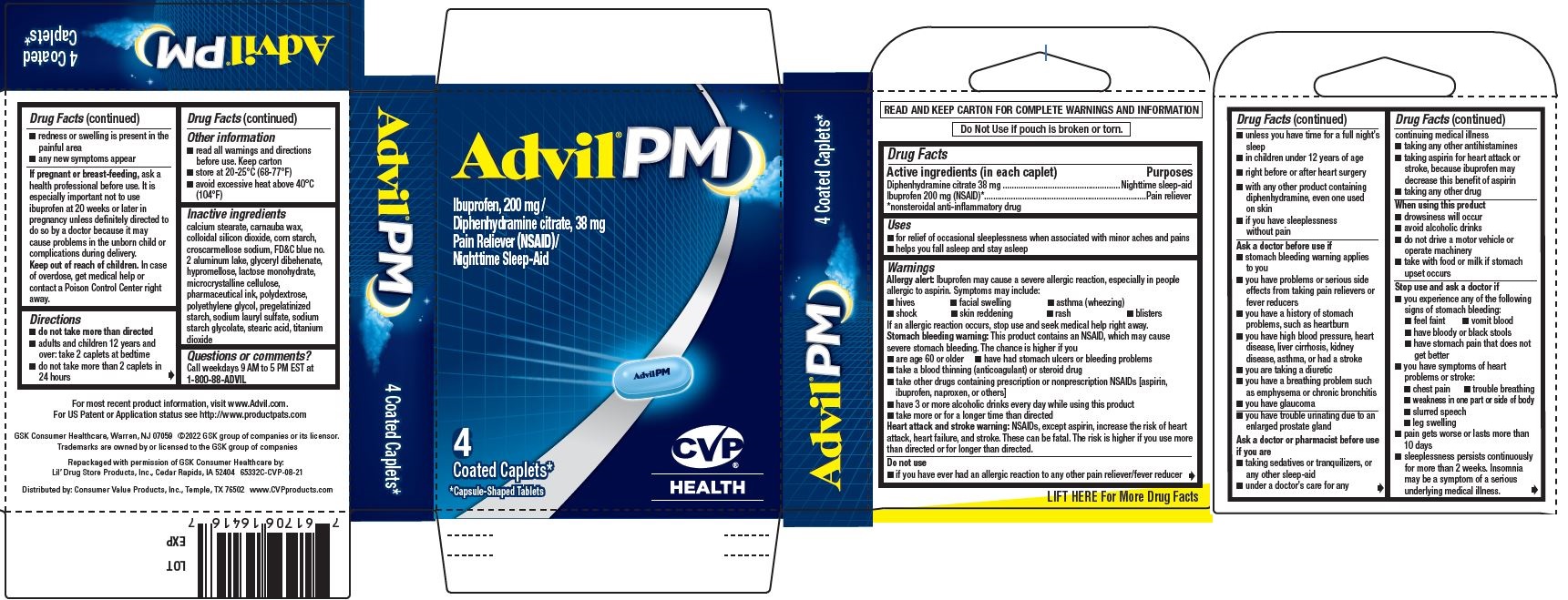
Uses
- for relief of occasional sleeplessness when associated with minor aches and pains
- helps you fall asleep and stay asleep
Warnings
Allergy alert
Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Heart attack and stroke warning
NSAIDs, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
Do not use
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- unless you have time for a full night's sleep
- in children under 12 years of age
- right before or after heart surgery
- with any other product containing diphenhydramine, even one used on skin
- if you have sleeplessness without pain
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have problems or serious side effects from taking pain relievers or fever reducers
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, or had a stroke
- you are taking a diuretic
- you have a breathing problem such as emphysema or chronic bronchitis
- you have glaucoma
- you have trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers, or any other sleep-aid
- under a doctor's care for any continuing medical illness
- taking any other antihistamines
- taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- taking any other drug
When using this product
- drowsiness will occur
- avoid alcoholic drinks
- do not drive a motor vehicle or operate machinery
- take with food or milk if stomach upset occurs
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- you have symptoms of heart problems or stroke:
- chest pain
- trouble breathing
- weakness in one part or side of body
- slurred speech
- leg swelling
- pain gets worse or lasts more than 10 days
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
- redness or swelling is present in the painful area
- any new symptoms appear
Directions
- do not take more than directed
- adults and children 12 years and over: take 2 caplets at bedtime
- do not take more than 2 caplets in 24 hours
Other information
- read all warnings and directions before use. Keep carton
- store at 20-25°C (68-77°F)
- avoid excessive heat above 40°C (104°F)
Inactive ingredients
calcium stearate, carnauba wax, colloidal silicon dioxide, corn starch, croscarmellose sodium, FD&C blue no. 2 aluminum lake, glyceryl behenate, hypromellose, lactose monohydrate, microcrystalline cellulose, pharmaceutical ink, polydextrose, polyethylene glycol, pregelatinized starch, sodium lauryl sulfate, sodium starch glycolate, stearic acid, titanium dioxide
Repackaged and distributed with permission of Pfizer Consumer Healthcare by:
Lil' Drug Store Products, Inc., 1201 Continental Place NE, Cedar Rapids, IA 52402
Advil PM, 6ct, Lil' Drug Store ® - PDP/Package
Advil ® PM
Ibuprofen, 200 mg/
Diphenhydramine citrate, 38 mg
Pain Reliever (NSAID)/
Nighttime Sleep-Aid
[caplet image]
6
Coated Caplets*
*Capsule-Shaped Tablets
[ Lil' Drug Store ® logo]