Keep out of reach of children.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately (1-800-222-1212).
Ask a doctor before use if you have
- abdominal pain, nausea or vomiting.
- a sudden change in bowel habits lasting for more than 2 weeks.
- already used a laxative for more than 1 week.
Stop use and ask a doctor
if you have rectal bleeding or fail to have a bowel movement after using a laxative.
This may indicate a serious condition.
Directions
Single Daily dosage: 1 suppository or as directed by a doctor
- Adults and children 6 years and over:
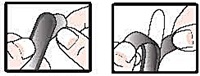
- detach 1 suppository from the strip; remove the wrapper before inserting into the rectum as follows:
- hold suppository with pointed end up
- carefully separate tabs with fingernail
- slowly pull apart by pulling tabs down on both sides
- remove suppository from wrapper
- insert one suppository well up into the rectum
- children 2 to 6 years use Leader Pediatric Glycerin Suppositories
- children under 2 years of age ask a doctor
Other information
- This product usually produces a bowel movement in 1/4 to 1 hour
- Store container tightly closed
- Keep from excessive heat
Adult Glycerin Suppositories Label
NDC 49718-081-25
Leader®
Compare to Fleet® active ingredient*
Glycerin Suppositories
Laxative
SATISFACTION GUARANTEED
25 Adult Size
TAMPER-EVIDENT FOR YOUR SAFETY, SUPPOSITORIES ARE PACKED IN TAMPER-EVIDENT SEALED WRAPPER. DO NOT USE IF WRAPPER IS TORN OR OPEN
E-Z Open Wrapper
Distributed by Cardinal Health, Inc.
Dublin, OH 43017 CIN # 4866216
www.myleader.com 1-800-200-6313
ALL LEADER® Brand Products are 100% satisfaction guaranteed or return to place of purchase for a full refund.
This product is not manufactured or distributed by C.B. Fleet Co. Inc., owner of the registered trademark Fleet®
