NATRUM SULPHURICUM- natrum sulphuricum tablet, chewable
HOMEOLAB USA INC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
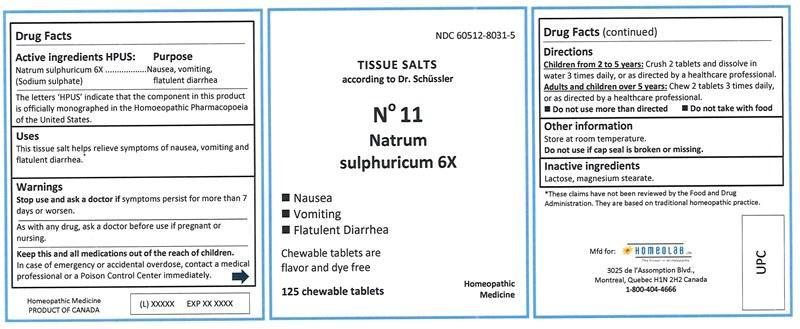
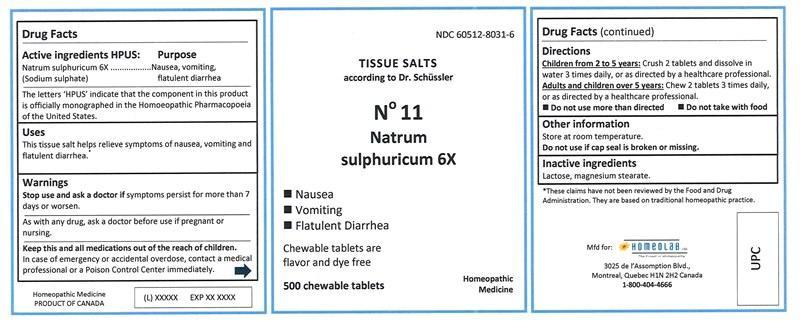
Active ingredients HPUS
Natrum sulphuricum (Sodium sulphate) 6X
Purpose
...........................................Nausea, vomiting, flatulent diarrhea
The letters 'HPUS' indicate that the component in this product is officially monographed in the Homoeopathic Pharmacopoeia of the United States.
Uses
This tissue salt helps relieve symptoms of nausea, vomiting and flatulent diarrhea.*
*These claims have not been reviewed by the Food and Drug Administration. They are based on traditional homeopathic practice.
Warnings
Stop use and ask a doctor if symptoms persist for more than 7 days or worsen.
As with any drug, ask a doctor before use if pregnant or nursing.
Keep this and all medications out of the reach of children.
In case of emergency or accidental overdose, contact a medical professional or a Poison Control Center immediately.
Directions
Children from 2 to 5 years: Crush 2 tablets and dissolve in water 3 times daily, or as directed by a healthcare professional.
Adults and children over 5 years: Chew 2 tablets 3 times daily, or as directed by a healthcare professional.
• Do not use more than directed.
• Do not take with food.
Other information
Store at room temperature.
Do not use if cap seal is broken or missing.
Inactive ingredients
Lactose, magnesium stearate.
LABEL