Keep out of reach of children
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
When using this product
skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Avoid contact with eyes. If contact occurs, flush thoroughly with water.
Directions
- Clean the skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day
Inactive ingredients
Water, poloxamer 407, propylene glycol, PEG-400, laureth-4, menthol, bisabolol.
BRODA Acne Spot Treatment and Acne Treatment Pen Labels
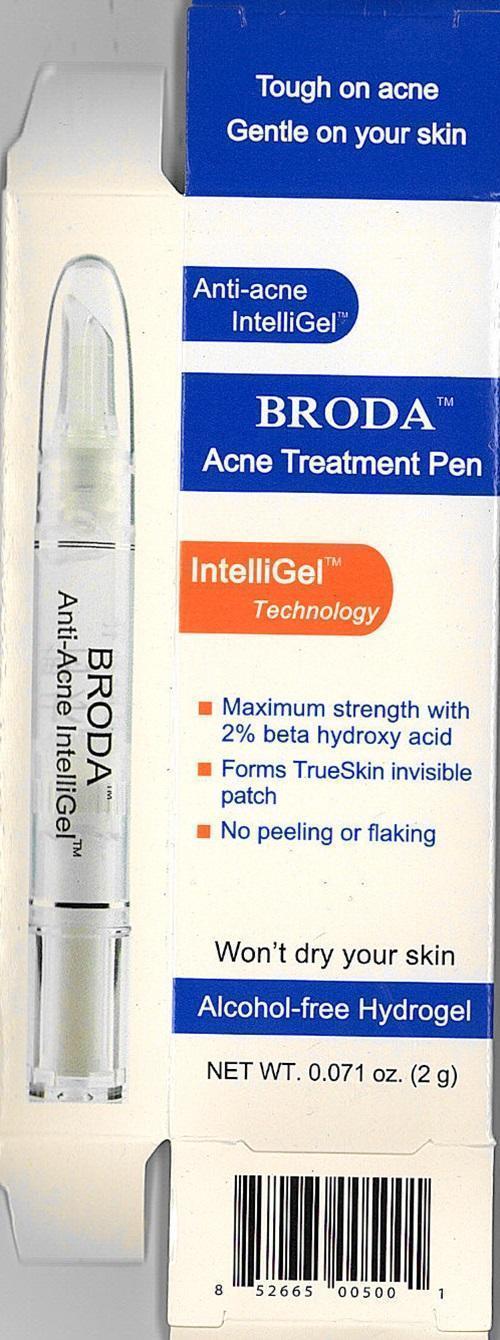
Tough on acne
Gentle on your skin
Anti-acne IntelliGel™
BRODA Acne Spot Treatment
Broda Acne Treatment Pen
IntelliGel™ Technology
Suitable for sensitive skin
Maximum strength with 2% beta hydrox acid
Forms TrueSkin invisible patch
No peeling or flaking
Won't dry your skin
Alcohol-free Hydrogel
NET WT. 0.071 oz. (2g) - 0.71 oz. (20 g)
Distributed by:
Broda International, LLC
10 Barley Court
Plainsboro, NJ 08536
Manufactured and packaged in the USA
www.broda.com
Patent pending
BRODA™
Anti-acne IntelliGel™
Developed with a proprietary break-through IntellGel™ technology that delivers maximum strength acne medication to effectively penetrate pores to clear most acne blemishes and help prevent new acne pimples.


res