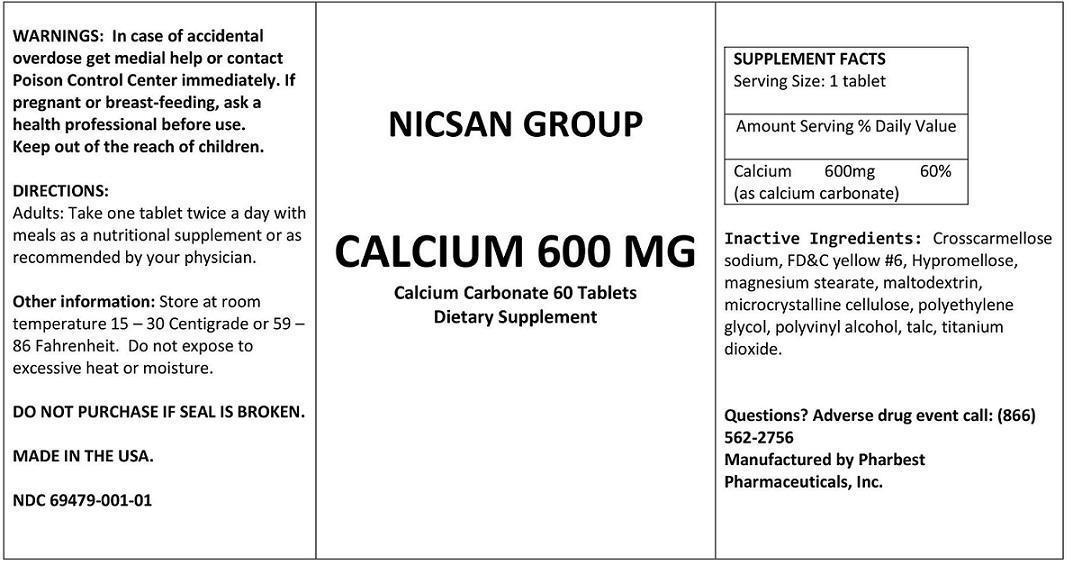
| SUPPLEMENT FACTS
Serving Size: 1 tablet | ||
| Amount Serving | % Daily Value | |
|
Calcium (as calcium carbonate) | 600mg | 60% |
Inactive Ingredients: Crosscarmellose sodium, FD&C yellow #6, Hypromellose, magnesium stearate,
maltodextrin, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide.
WARNINGS: In case of accidental overdose get medial help or contact Poison Control Center immediately.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of the reach of children.
Other information: Store at room temperature 15 – 30 Centigrade or 59 – 86 Fahrenheit. Do not expose to excessive heat or moisture.
Questions? Adverse drug event call:(866) 562-2756