ANTIBACTERIAL WET WIPES- benzalkonium chloride swab
Zhejiang Qimei Commodity Co.,Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
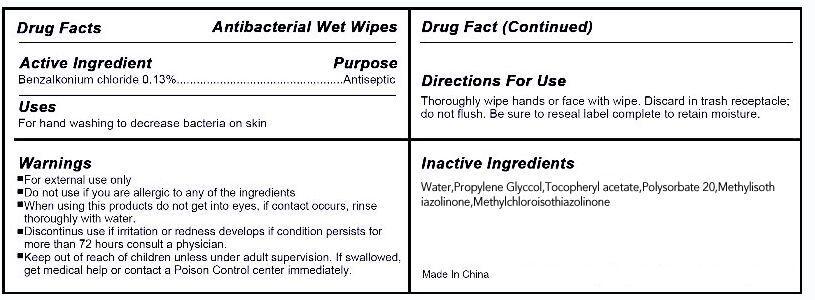
Active ingredient
benzalkonium chloride 0.13%
Uses
for hand washing to decrease bacteria on skin
Warnings
For External use only.
Do not use if you are allergic to any of the ingredients.
When using this product Do not get into eyes,if contact occurs ,rinse throughly with water.
Discontinue use if irritation or redness develops if condition persists for more than 72 hours consults a physician.
Keep out of reach of children
keep out of reach of children unless under adult supervision .if seallowed ,get medical help or contact a Poison Control Center immediately.
Directions
Thoroughly wipe hands or face with wipe,Discard in trash receptacle;do not flush.Be sure to reseal label complete to retain moisture
WATER PROPYLENE GLYCOL OCTHILINONE .ALPHA.-TOCOPHEROL
Zhejiang Qimei Commodity Co.,Ltd.