Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes.
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if you prescription contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Directions
- do not take more than 6 doses in any 24-hour period
- this adult product is not intended for use
in children under 12 years of age -
adults and children 12 years of age and over
2 teaspoons every 4 hours - children under 12 years do not use
Other information
- each teaspoon contains: sodium 7 mg
- store 20 to 25°C (68 to 77°F)
- dosage cup provided
Inactive Ingredients
anhydrous citric acid, FD&C red no.40, glycerin, high fructose corn syrup, menthol, natural flavor, propylene glycol, purified water, sodium benzoate, sodium citrate, sucralose
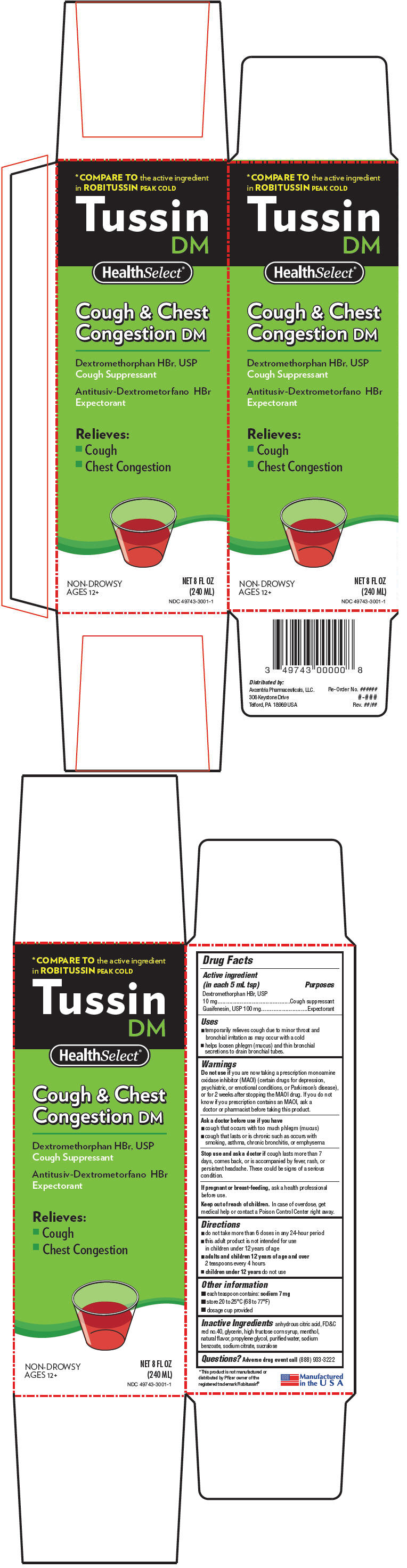
PRINCIPAL DISPLAY PANEL - 240 ML Bottle Carton
*COMPARE TO the active ingredient
in ROBITUSSIN PEAK COLD
Tussin
DM
HealthSelect®
Cough & Chest
Congestion DM
Dextromethorphan HBr, USP
Cough Suppressant
Antitusiv-Dextrometorfano HBr
Expectorant
Relieves:
- Cough
- Chest Congestion
NON-DROWSY
AGES 12+
NET 8 FL OZ
(240 ML)
NDC 49743-3001-1