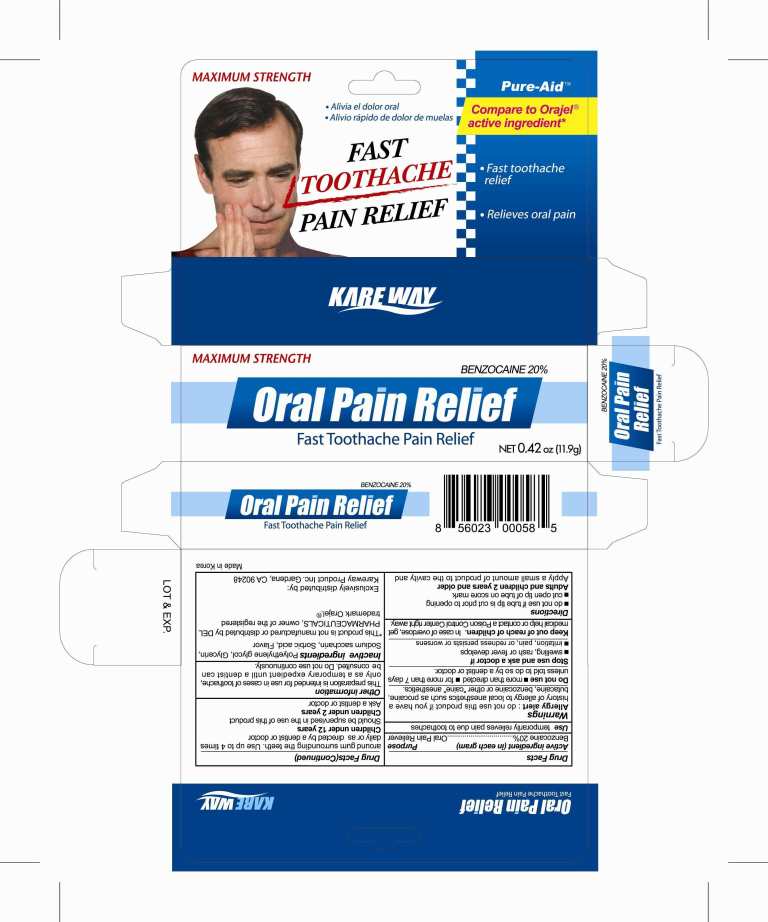
Warnings
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics
Directions
Directions do not use if tube tip is cut prior to opening; cut open tip of tube on score mark
Adults and children 2 years of age and over Apply a small amount of product to the cavity and around gum surrounding the teeth.
Use up to 4 times daily or as directed by a dentist or doctor
Children under 12 years of age Should be supervised in the use of this product
Children under 2 years of age Ask a dentist or doctor
Other information
This preparation is intended for use in case of toothache, only as a temporary expedient until a dentist can be consulted;
do not use continuously